Abstract
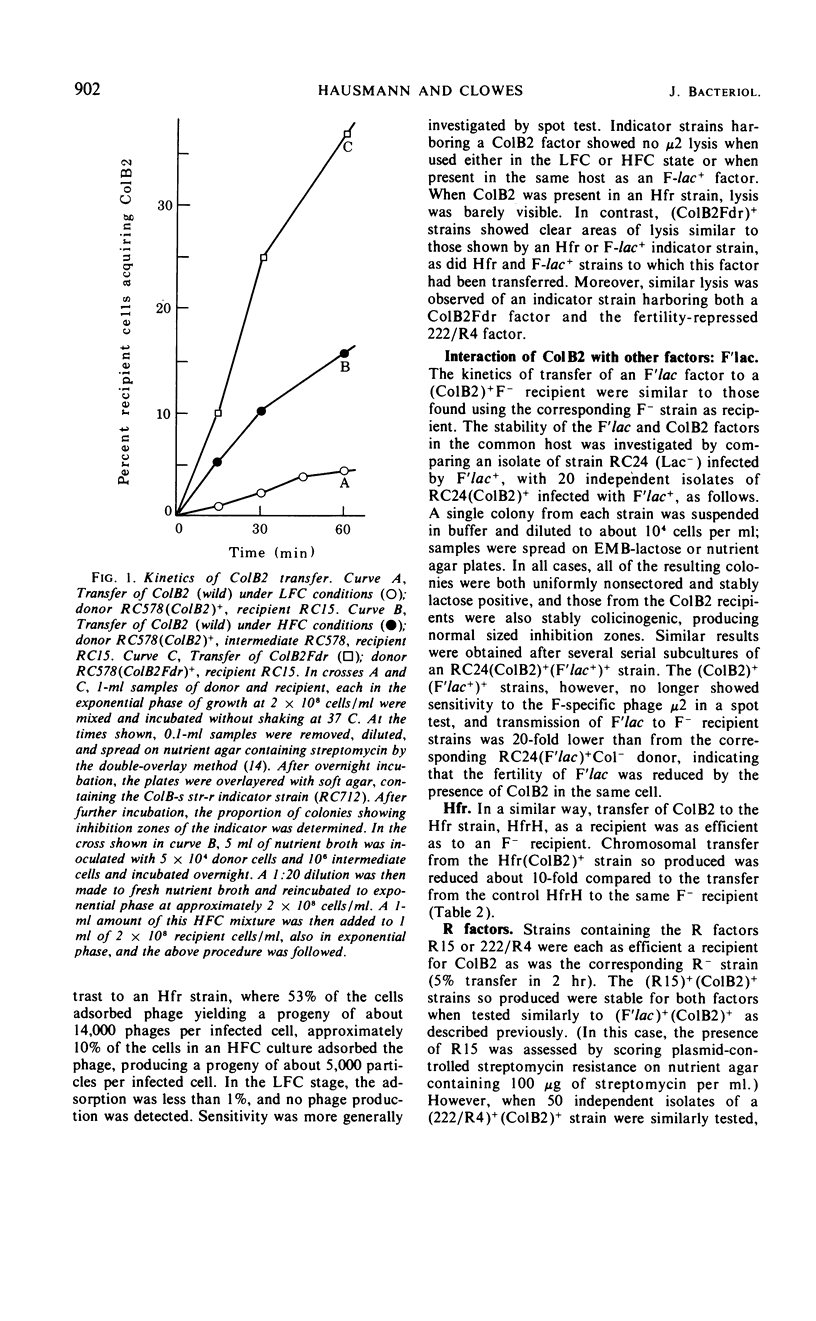
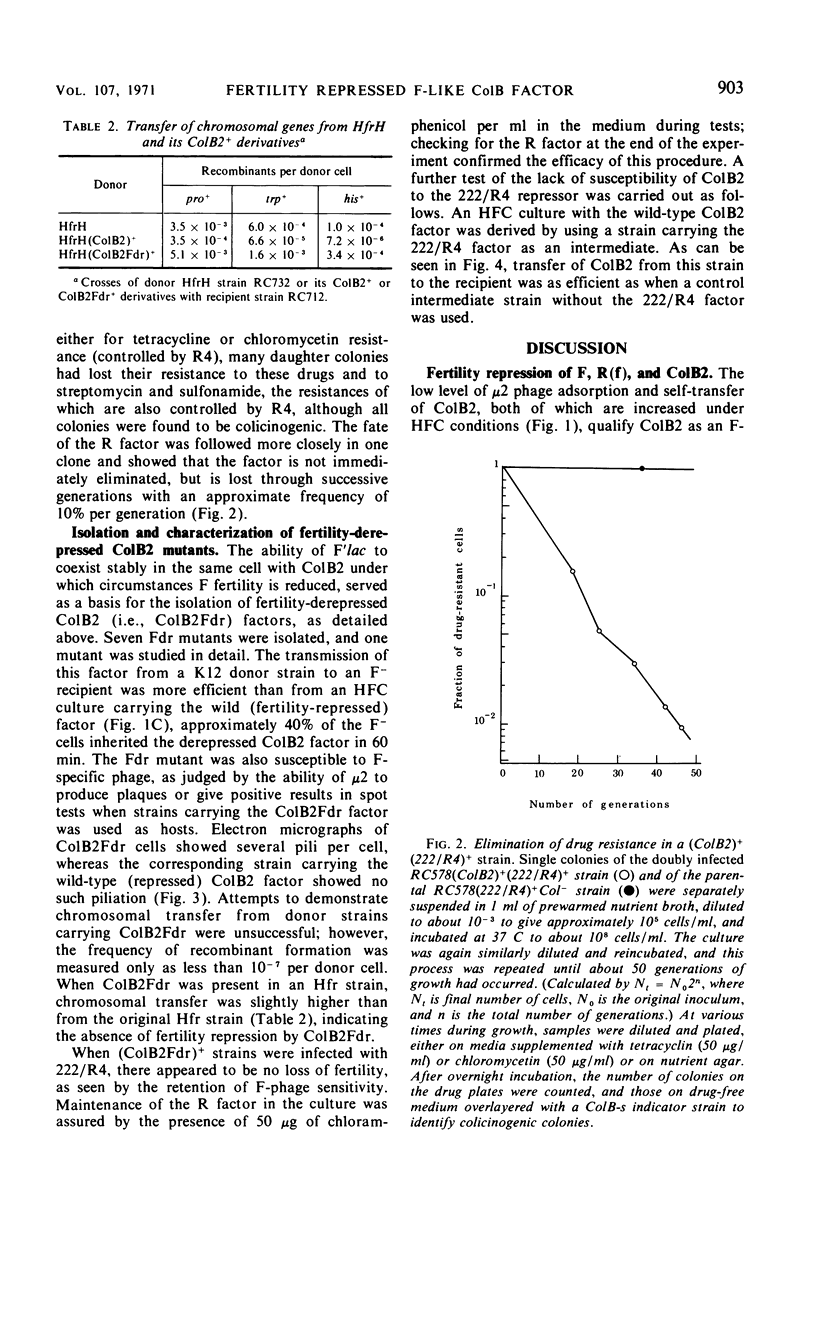
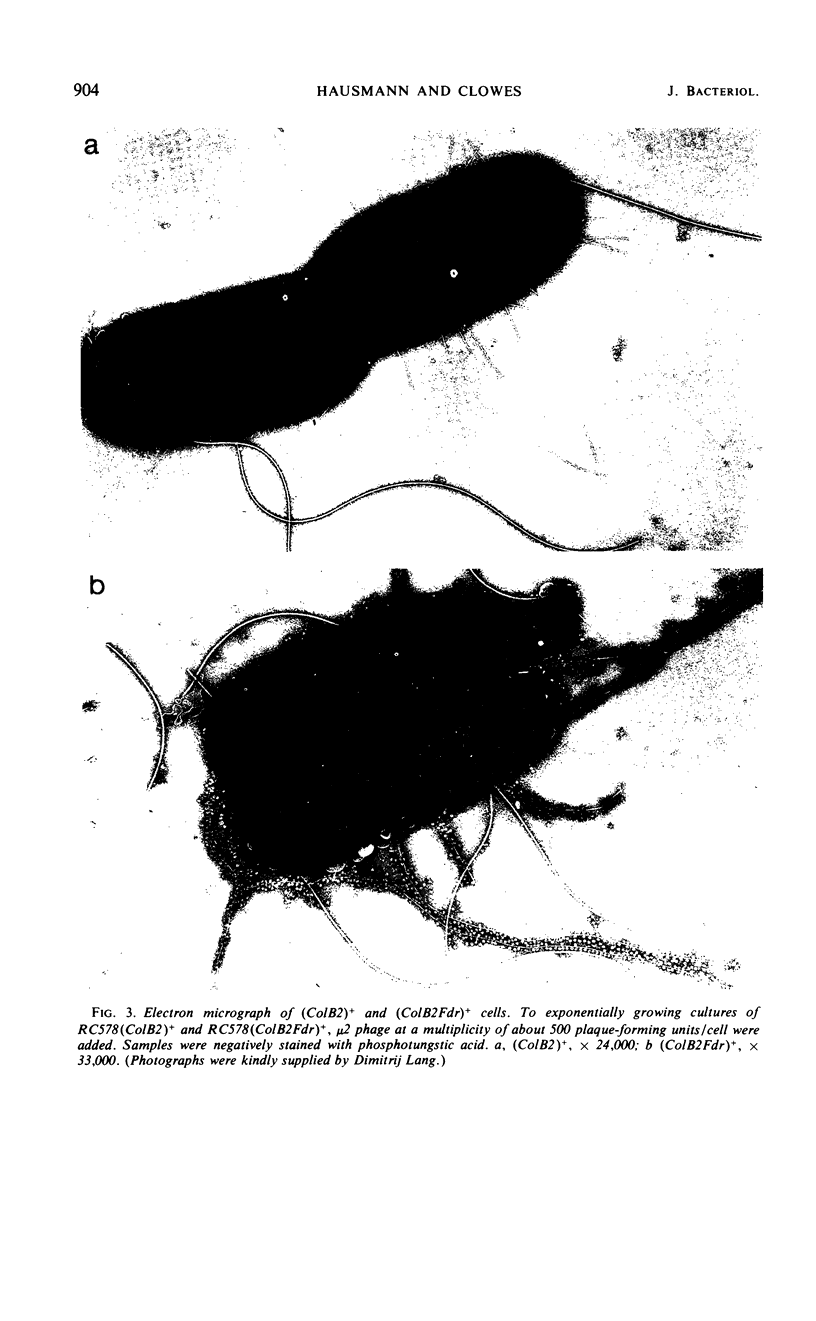
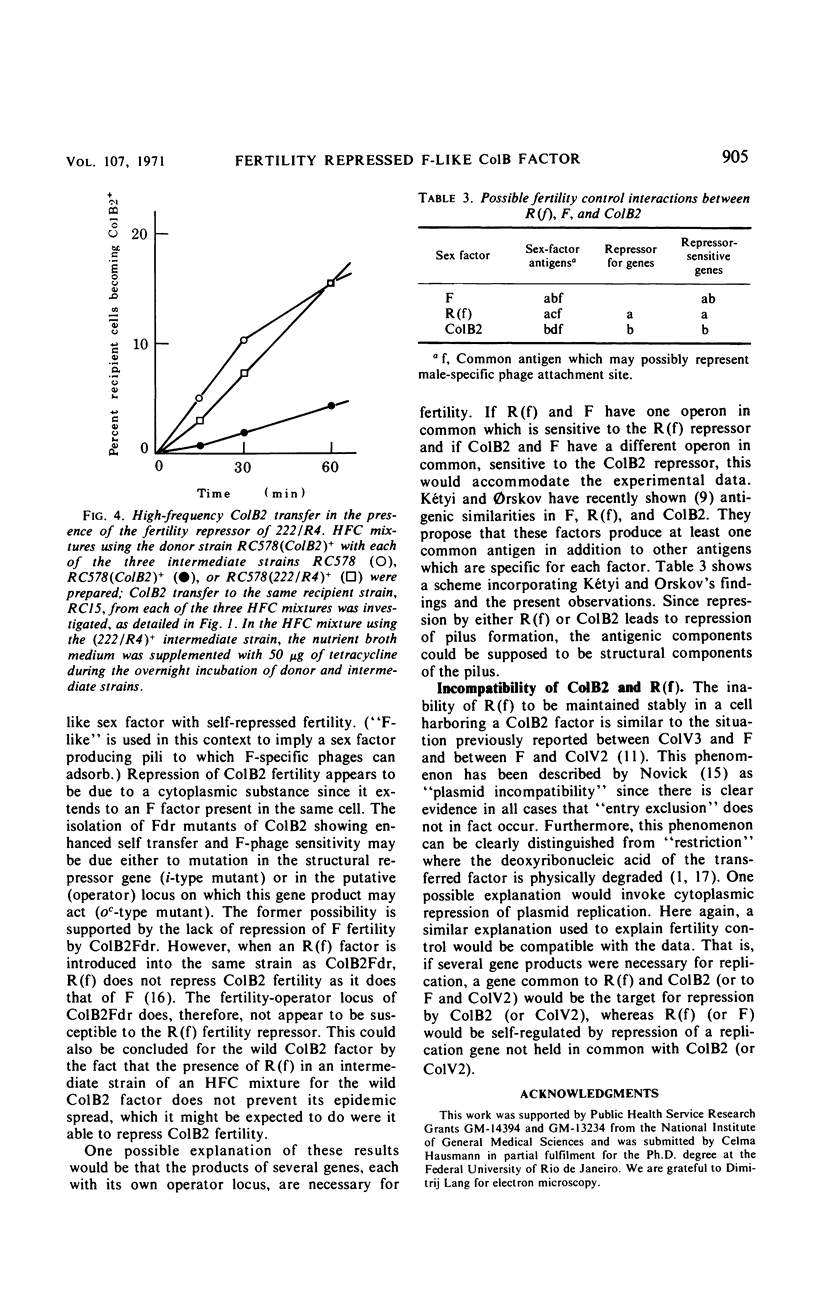
The colicinogenic B factor, transferred from Escherichia coli strain K77 (and termed ColB2-K77 or ColB2) to an E. coli K12 F− strain, is capable of promoting its own transfer to other K12 F− strains at a low rate (from LFC cultures) which can be increased under special conditions (HFC cultures). LFC cultures of K12 (ColB2)+ F− strains show a low level of adsorption of F-specific phage particles which also increases under HFC conditions. The ColB2 factor is thus inferred to be an F-like sex factor which is repressed in its fertility. This repression is concluded to be due to a cytoplasmic repressor since, when ColB2 is present in cells containing an F factor (either autonomous or integrated), F fertility is also repressed as shown by the inability of such (ColB2)+F+ [or (ColB2)+Hfr] strains to plaque F-specific phages, and by a reduction in the level of chromosomal transfer from such strains, compared to the corresponding F+ (or Hfr) control strains. Mutants of the ColB2 factor in which fertility is no longer repressed (fertility derepressed or Fdr mutants) have been isolated. The ColB2Fdr mutant strains do not appear to be able to mobilize chromosomal transfer, although they have acquired F-specific phage sensitivity demonstrable by plaque formation and they transfer their colicin factor at high frequency and are well piliated. The Fdr mutation is presumed to result in the inability to synthesize the cytoplasmic fertility repressor since the ColB2Fdr factor does not repress the fertility of an F factor when present in the same host strain. A fertility-repressed drug resistance factor of the R(f) type is not stable in the presence of a ColB2 factor in the same cell and is eliminated in about 10% of the cells per generation. In contrast, another factor characteristic of the R(i) type is fully compatible with ColB2. Under conditions artificially stabilizing (ColB2Fdr)+ (Rf)+ strains, the enhanced fertility of ColB2Fdr is not repressed by the presence of the R factor, nor does the presence of R(f) in the intermediate strain of an HFC (for ColB2) system inhibit the normal increase in ColB2 transmissibility. It is concluded that the repressors of R(f) and ColB2, although both active on F fertility, are different; this may indicate that at least two independently repressible cistrons are involved in the expression of fertility characteristics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Edwards S., Meynell G. G. General method for isolating de-repressed bacterial sex factors. Nature. 1968 Aug 24;219(5156):869–870. doi: 10.1038/219869a0. [DOI] [PubMed] [Google Scholar]
- Frydman A., Meynell E. Interactions between de-repressed F-like R factors and wild type colicin B factors: superinfection immunity and repressor susceptibility. Genet Res. 1969 Dec;14(3):315–322. doi: 10.1017/s0016672300002135. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kétyi I., Orskov I. Studies on the antigenic structure of sex fimbriae carried by a strain of Shigella flexneri 4b. Acta Pathol Microbiol Scand. 1969;77(2):299–308. doi: 10.1111/j.1699-0463.1969.tb04235.x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. TRANSFER OF THE COLICIN I FACTOR IN ESCHERICHIA COLI K12 AND ITS INTERACTION WITH THE F FERTILITY FACTOR. J Gen Microbiol. 1964 Sep;36:365–384. doi: 10.1099/00221287-36-3-365. [DOI] [PubMed] [Google Scholar]
- MacFarren A. C., Clowes R. C. A comparative study of two F-like colicin factors, ColV2 and ColV3, in Escherichia coli K-12. J Bacteriol. 1967 Aug;94(2):365–377. doi: 10.1128/jb.94.2.365-377.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]