Abstract
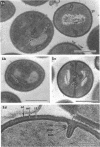
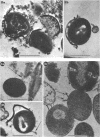
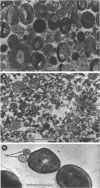
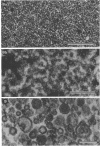
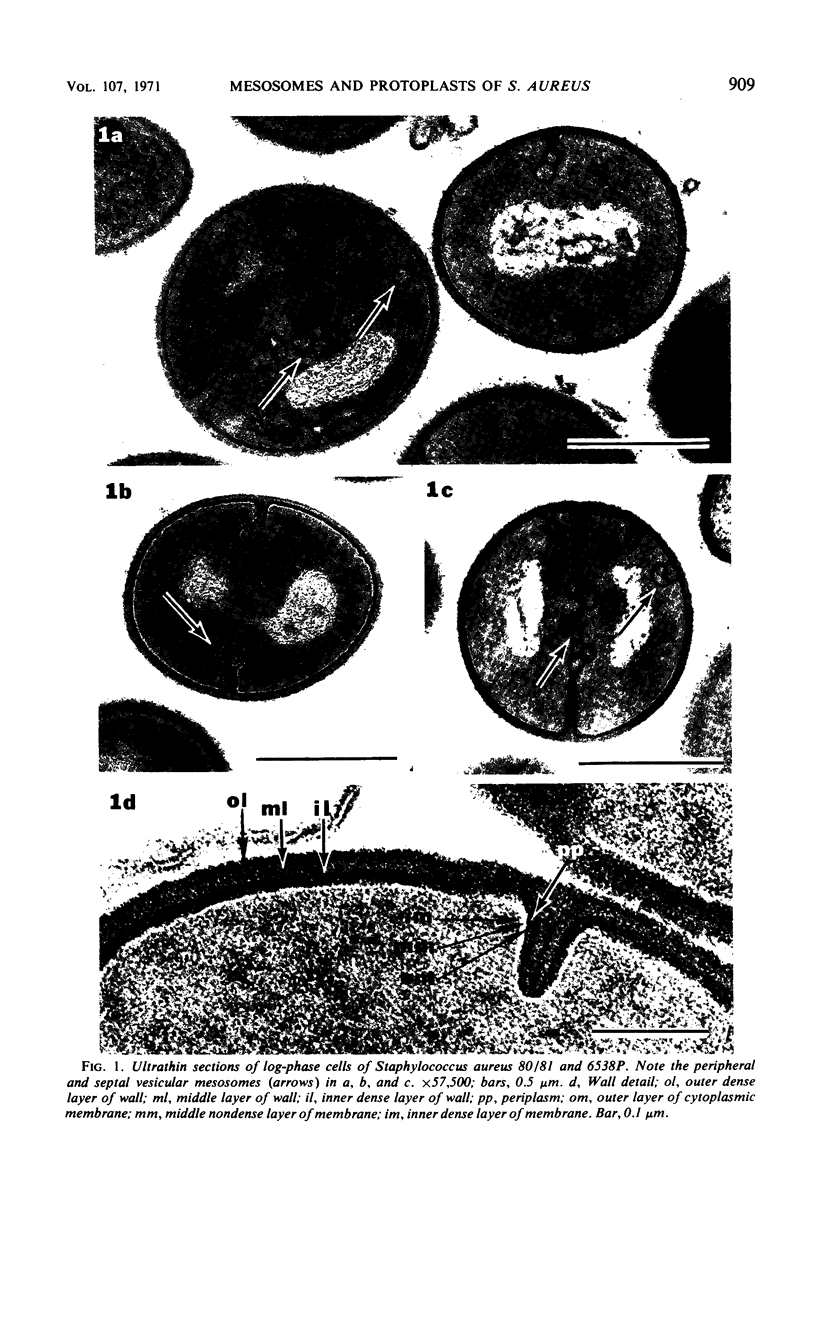
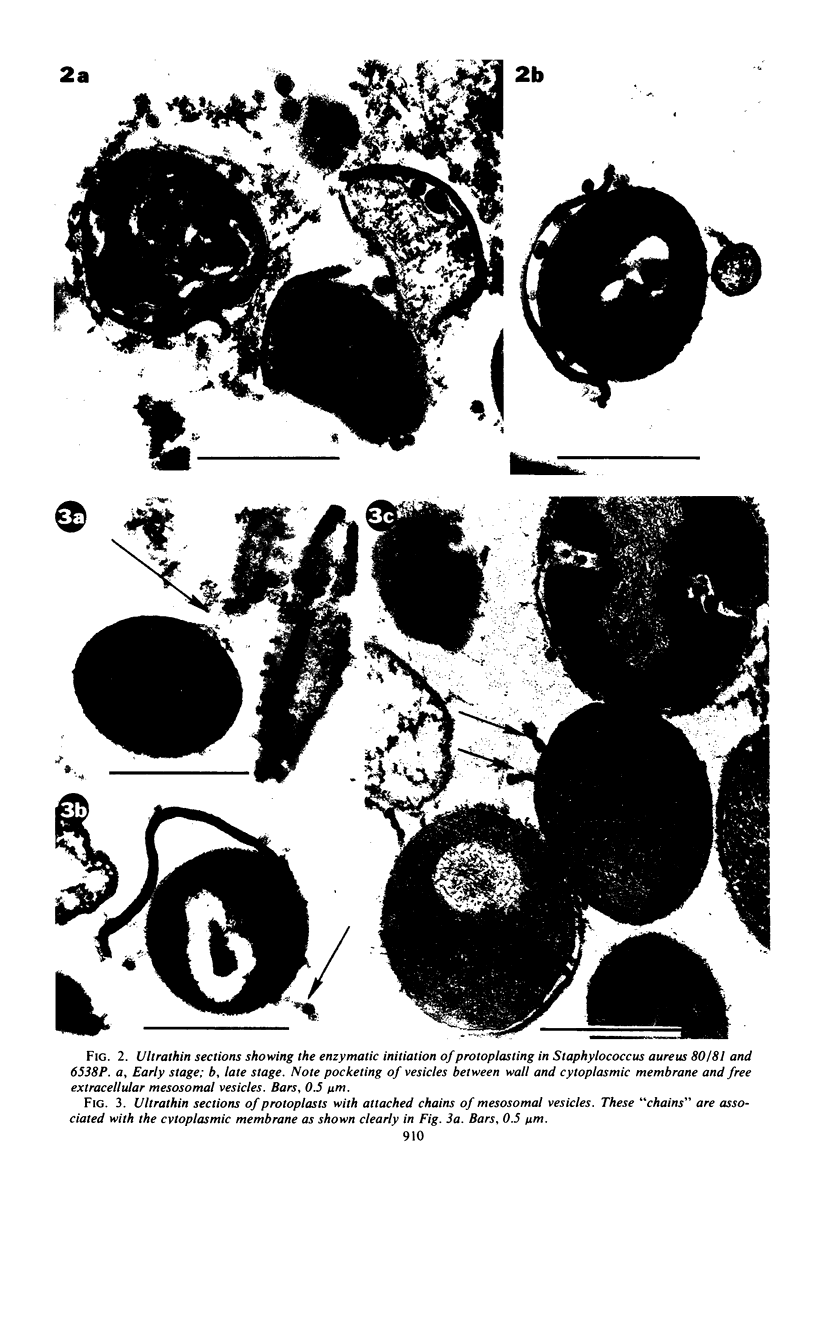
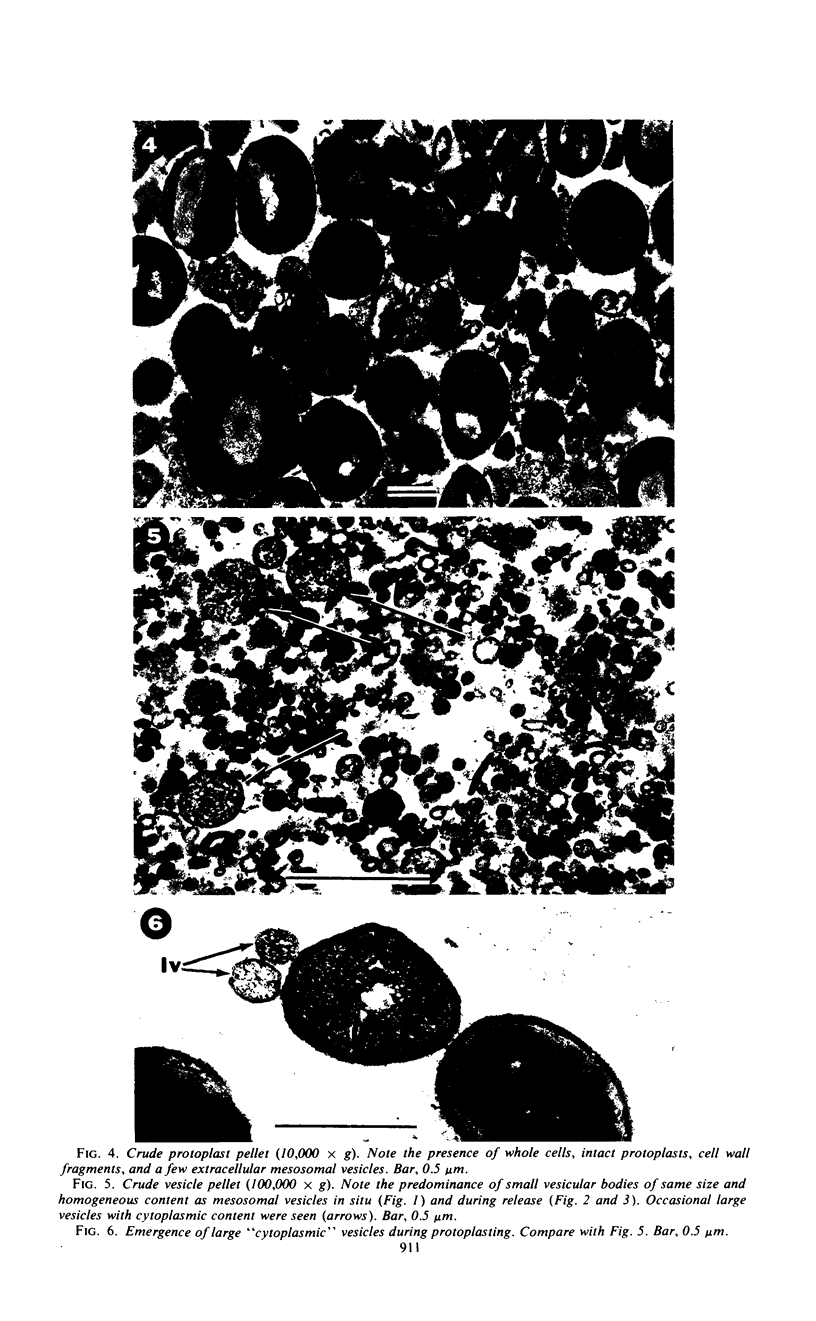
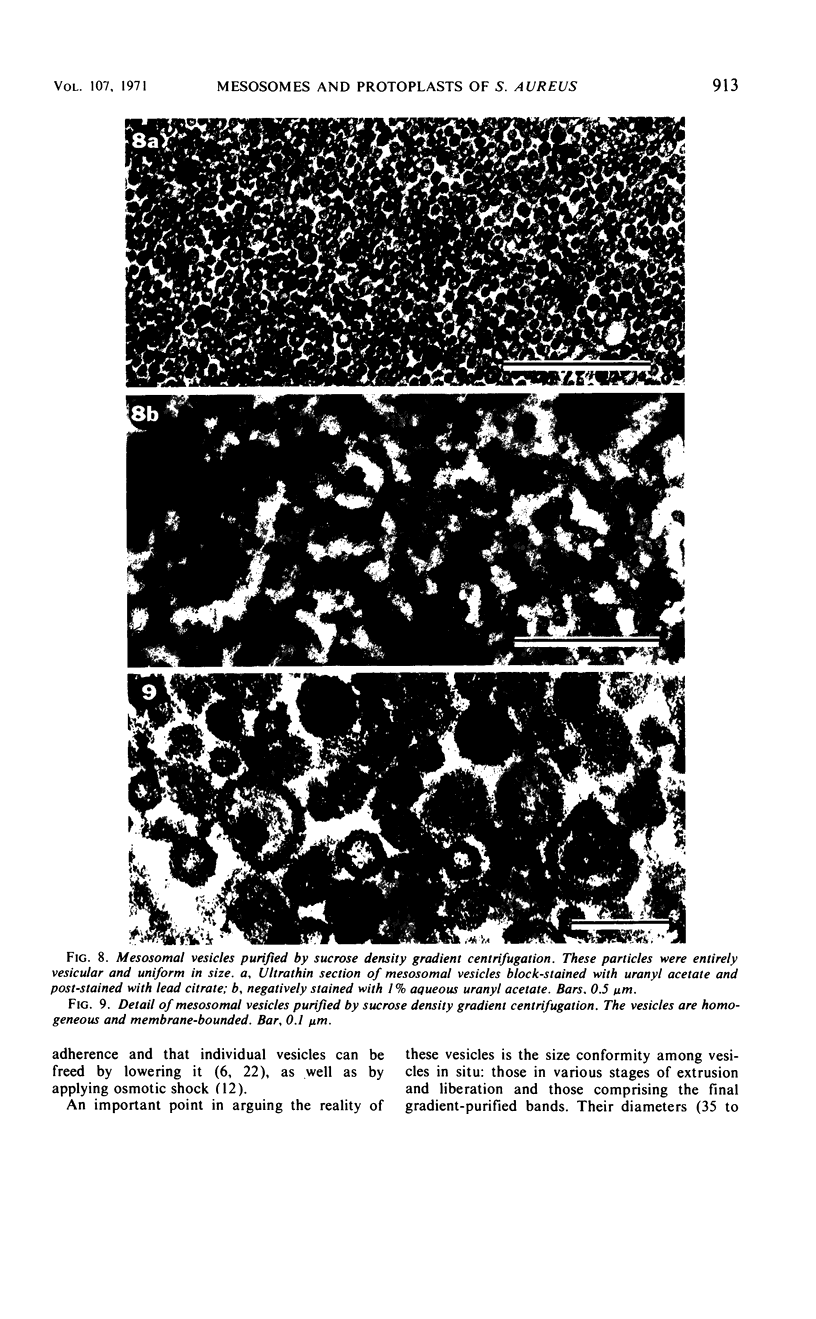
The mesosomes of log-phase Staphylococcus aureus ATCC 6538P and Staphylococcus aureus phage-type 80/81, as seen in situ in ultrathin sections, were of the vesicular type. The constituent vesicles ranged from 35 to 50 nm in diameter when the glutaraldehyde-osmium-uranium-lead sequence of fixation and staining was used. During protoplasting in hypertonic buffer containing a muralytic enzyme, vesicles of the same size were extruded and required magnesium ion to maintain structural integrity. The vesicles, purified from the protoplasting supernatant medium by density gradient centrifugation, maintained size and configuration in a homogeneous preparation. Cytoplasmic membranes, produced by osmotic shock and nuclease treatment of protoplasts, were similarly concentrated in gradients. However, they were not free of membrane-associated ribosomes nor of mesosomal vesicles except when prepared in the absence of magnesium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaton C. D. An electron microscope study of the mesosomes of a penicillinase-producing staphylococcus. J Gen Microbiol. 1968 Jan;50(1):37–42. doi: 10.1099/00221287-50-1-37. [DOI] [PubMed] [Google Scholar]
- Bulger R. J., Bulger R. E. Ultrastructure of small colony variants of a methicillin-resistant Staphylococcus aureus. J Bacteriol. 1967 Oct;94(4):1244–1246. doi: 10.1128/jb.94.4.1244-1246.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S. F., Jacobs N. J., Gray C. T. Ultrastructure and respiratory capacity of Staphylococcus and Bacillus grown under aerobic and anaerobic conditions. J Bacteriol. 1968 Aug;96(2):554–556. doi: 10.1128/jb.96.2.554-556.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Freer J. H. The isolation and characterisation of mesosome material from Micrococcus lysodeikticus. J Gen Microbiol. 1969 Nov;58(3):vii–vii. [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Chaix P., Ryter A. Localisation des cytochromes de Bacillus subtilis dans les structures mésosomiques. C R Acad Sci Hebd Seances Acad Sci D. 1966 Nov 21;263(21):1632–1635. [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fractionation and characterization of the plasma and mesosome membrane of Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):426–440. doi: 10.1128/jb.97.1.426-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M. Cellular site of Escherichia coli ribosomal RNA synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):435–439. doi: 10.1073/pnas.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of cell growth and mesosomal structure of Bacillus licheniformis. J Ultrastruct Res. 1969 Jan;26(1):130–147. doi: 10.1016/s0022-5320(69)90040-9. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawashige E. [Electron microscopic studies on the enzymatic activities in the membrane system of Staphylococci]. J Electron Microsc (Tokyo) 1967;16(4):293–303. [PubMed] [Google Scholar]
- Suganuma A. Electron microscopic studies of Staphylococci by serial sections. J Electron Microsc (Tokyo) 1968;17(4):315–321. [PubMed] [Google Scholar]
- Suganuma A. Further studies on the plasma membrane of Staphylococcus aureus. J Cell Biol. 1966 Jul;30(1):208–210. doi: 10.1083/jcb.30.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]