Abstract
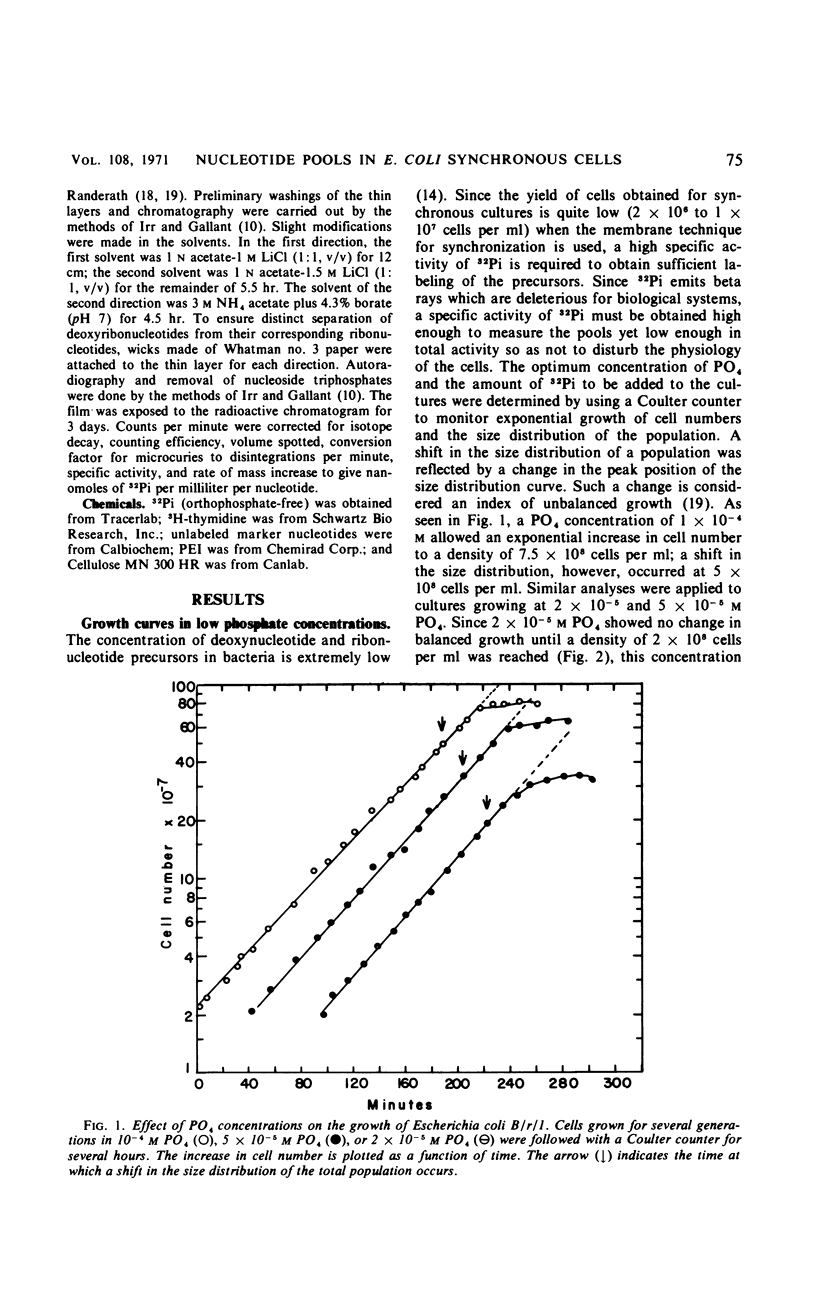
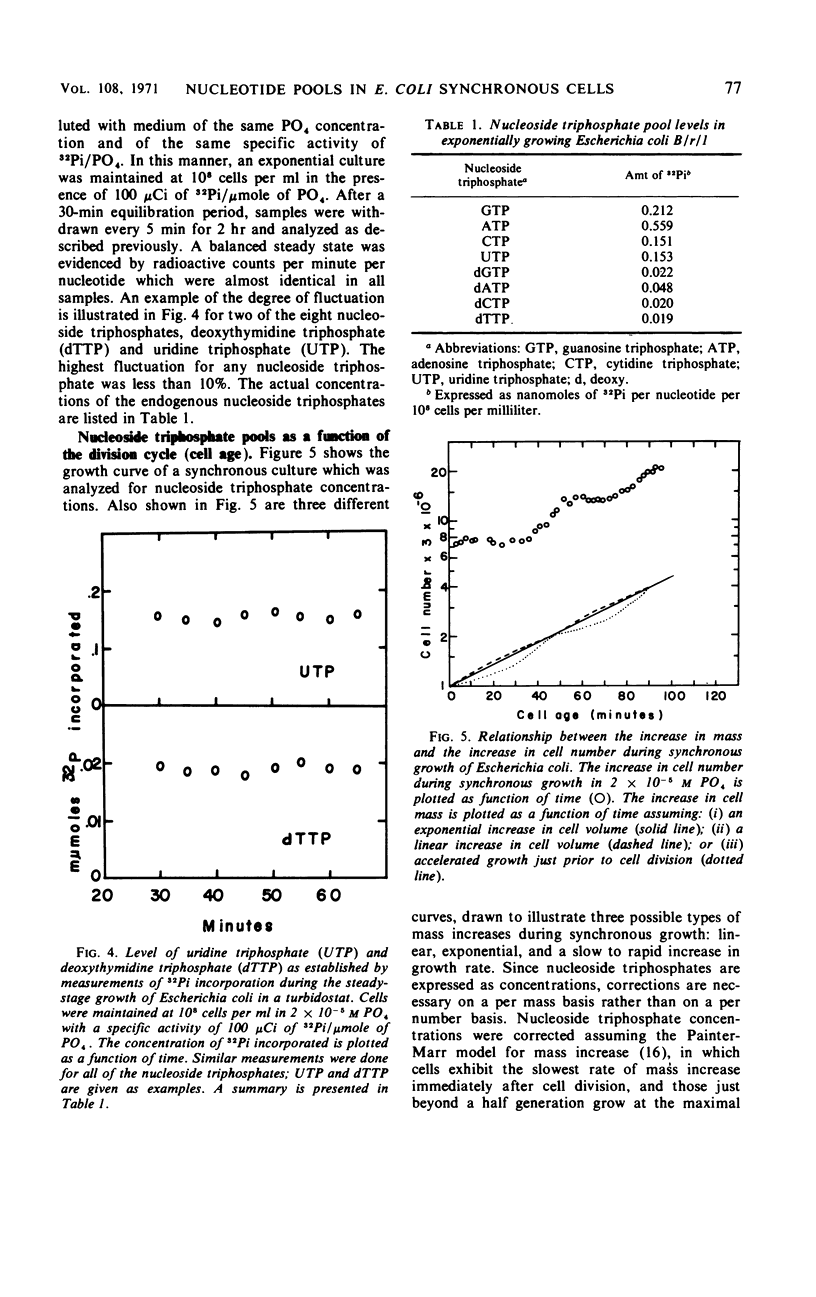
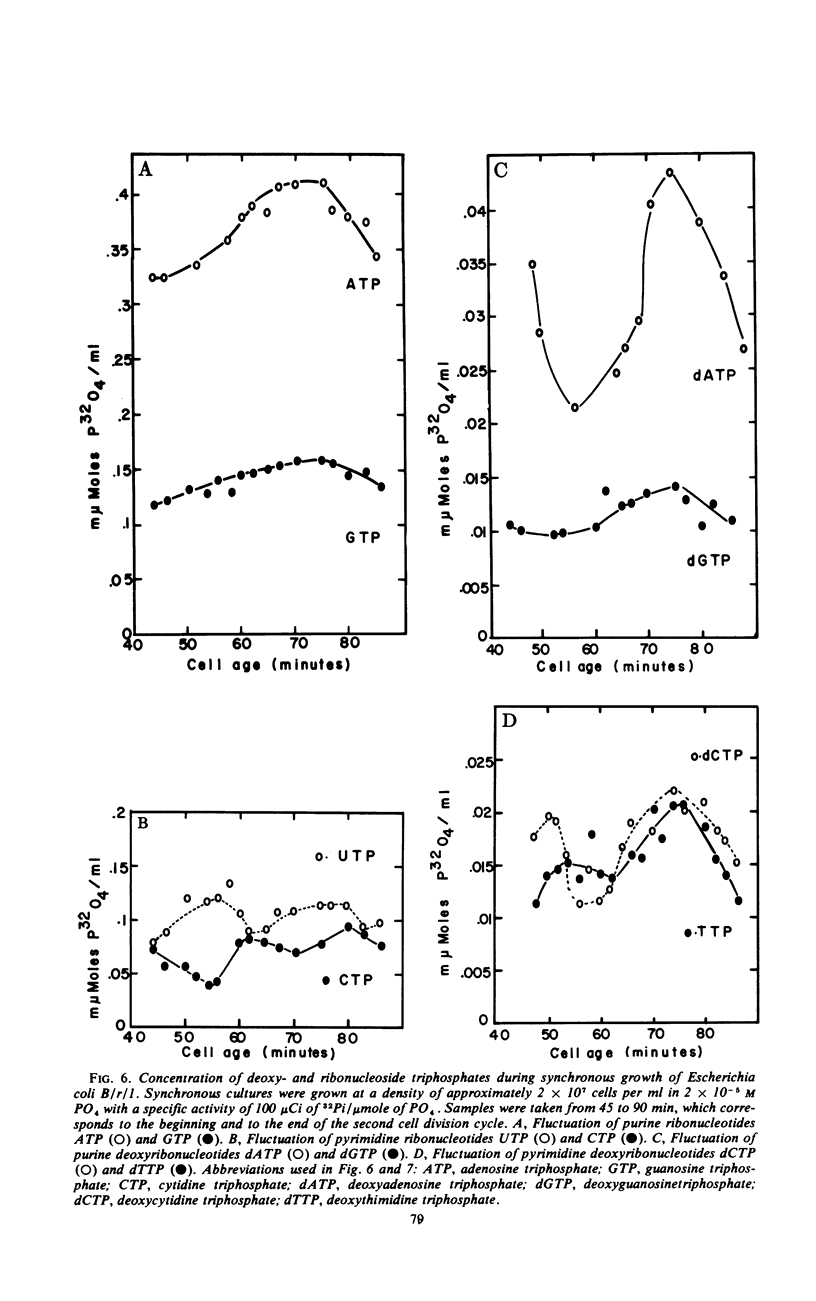
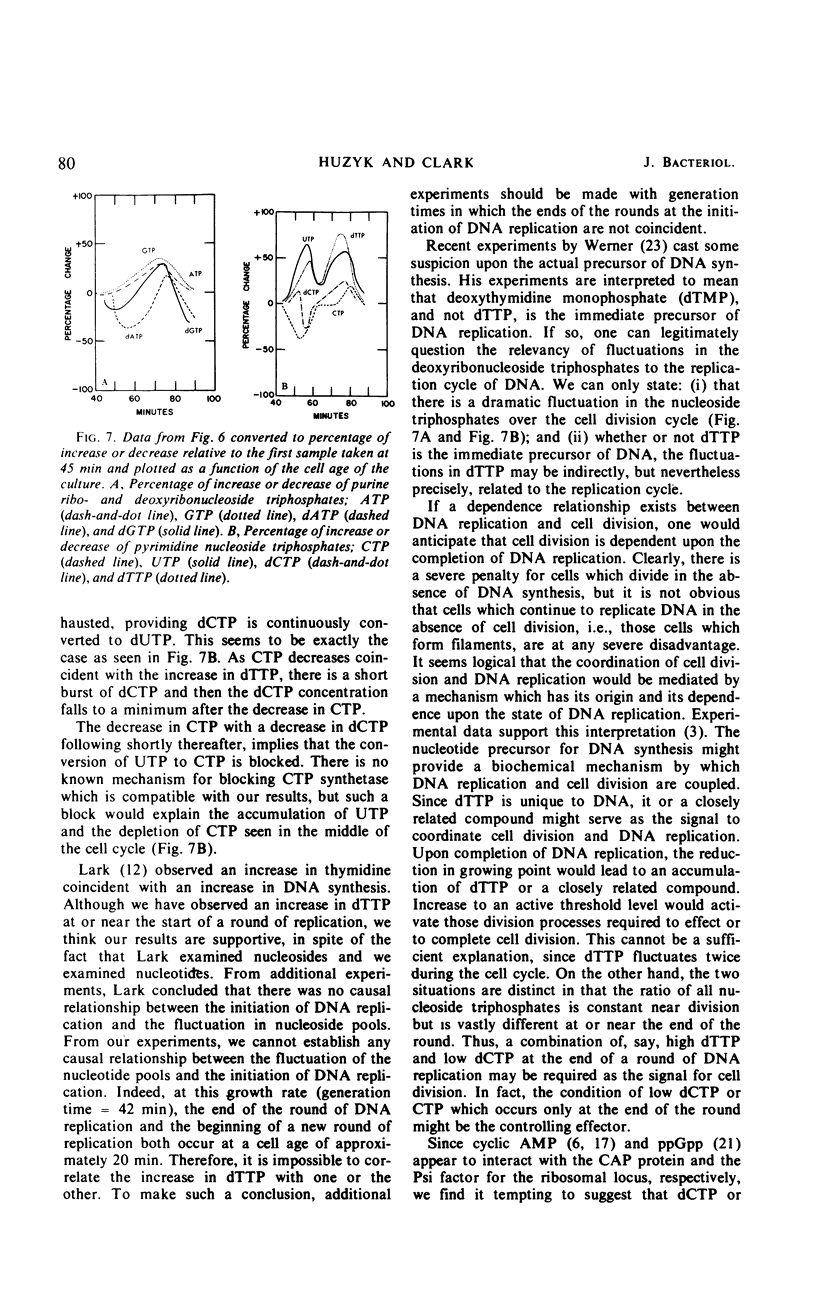
Endogenous nucleoside triphosphate pools in synchronized cultures of Escherichia coli B/r/1 oscillate as a function of age. Purine nucleoside triphosphates show a gradual 50% increase from zero age to the time of subsequent division, immediately prior to division. In contrast, pyrimidine nucleoside triphosphates undergo a dramatic change of about 50% in the first half of the generation at a time coincident with the termination of a round of deoxyribonucleic acid replication. A 50 to 70% increase starts at the initiation of the next round of deoxyribonucleic acid replication and continues until cell division, in parallel with the purine nucleotides. The fluctuation of pyrimidines between zero age and the middle of the division cycle suggests a functional relationship for pyrimidine metabolism and the regulation of cell division.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. C., Reichard P. Role of effector binding in allosteric control of ribonucleoside diphosphate reductase. J Mol Biol. 1969 Nov 28;46(1):39–55. doi: 10.1016/0022-2836(69)90056-4. [DOI] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Stent G. S. Nucleoside triphosphate pools and the regulation of RNA synthesis in E. coli. Proc Natl Acad Sci U S A. 1969 Feb;62(2):475–482. doi: 10.1073/pnas.62.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMSTETTER C. E., CUMMINGS D. J. AN IMPROVED METHOD FOR THE SELECTION OF BACTERIAL CELLS AT DIVISION. Biochim Biophys Acta. 1964 Mar 16;82:608–610. doi: 10.1016/0304-4165(64)90453-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Irr J., Gallant J. The control of ribonucleic acid synthesis in Escherichia coli. II. Stringent control of energy metabolism. J Biol Chem. 1969 Apr 25;244(8):2233–2239. [PubMed] [Google Scholar]
- Johnson R. A., Schmidt R. R. Enzymic control of nucleic acid synthesis during synchronous growth of Chlorella pyrenoidosa. I. Deoxythymidine monophosphate kinase. Biochim Biophys Acta. 1966 Oct 24;129(1):140–144. doi: 10.1016/0005-2787(66)90015-3. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. 3. On the regulation of the deoxyadenosine triphosphate and deoxycytidine triphosphate pools of Escherichia coli. Biochim Biophys Acta. 1966 Oct 24;129(1):104–115. doi: 10.1016/0005-2787(66)90012-8. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter P. R., Marr A. G. Mathematics of microbial populations. Annu Rev Microbiol. 1968;22:519–548. doi: 10.1146/annurev.mi.22.100168.002511. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Kamen R. I., Schleif R. F. Factor necessary for ribosomal RNA synthesis. Nature. 1970 Nov 21;228(5273):748–751. doi: 10.1038/228748a0. [DOI] [PubMed] [Google Scholar]
- Turner M. K., Abrams R., Lieberman I. Levels of ribonucleotide reductase activity during the division cycle of the L cell. J Biol Chem. 1968 Jul 10;243(13):3725–3728. [PubMed] [Google Scholar]
- Werner R. Mechanism of DNA replication. Nature. 1971 Apr 30;230(5296):570–572. doi: 10.1038/230570a0. [DOI] [PubMed] [Google Scholar]


