Abstract
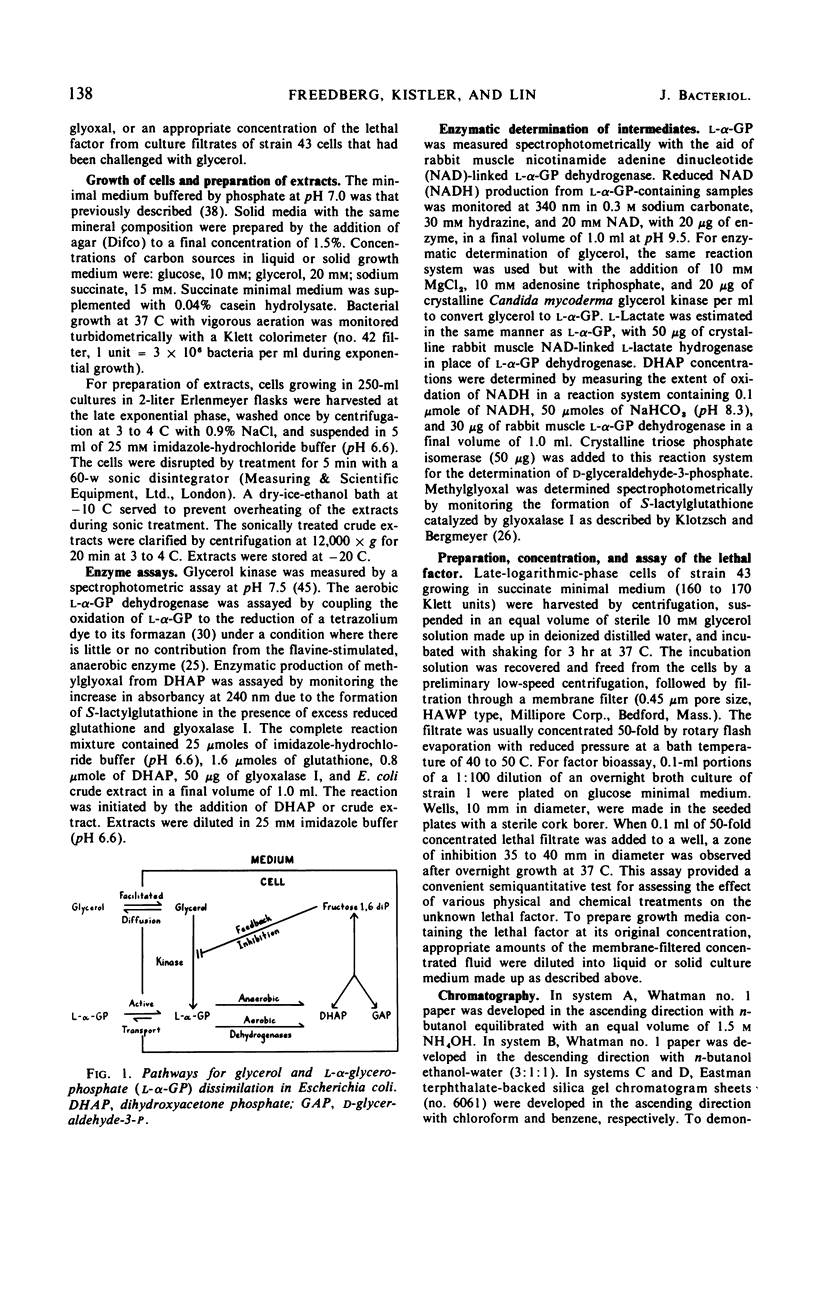
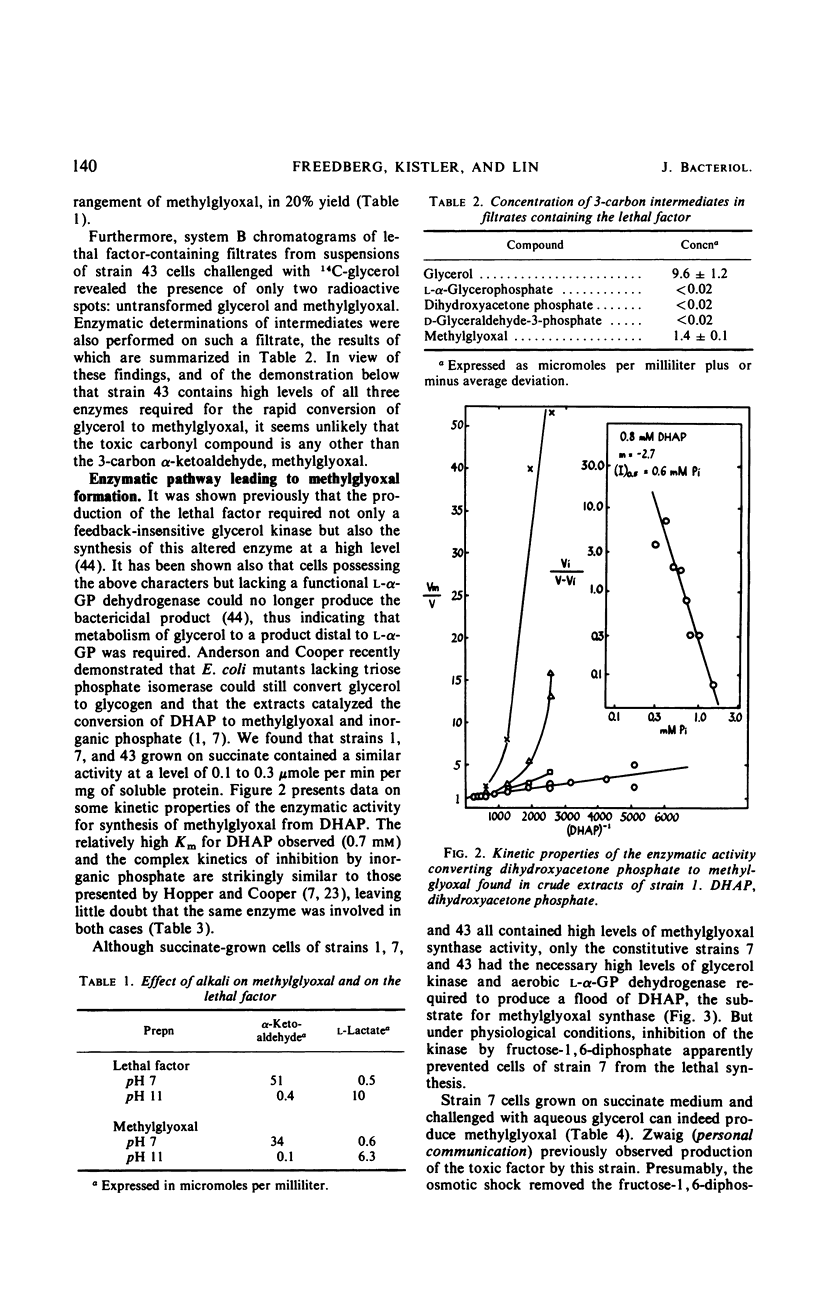
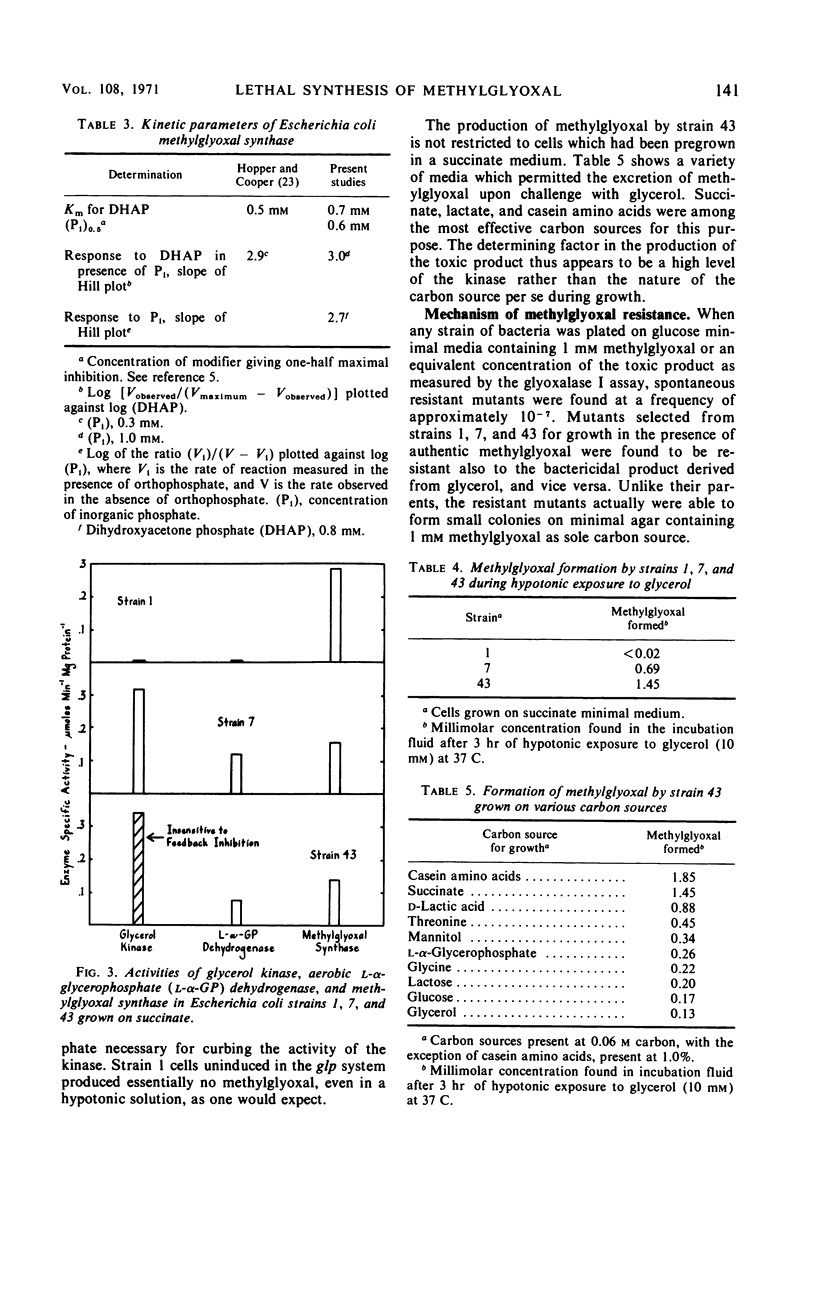
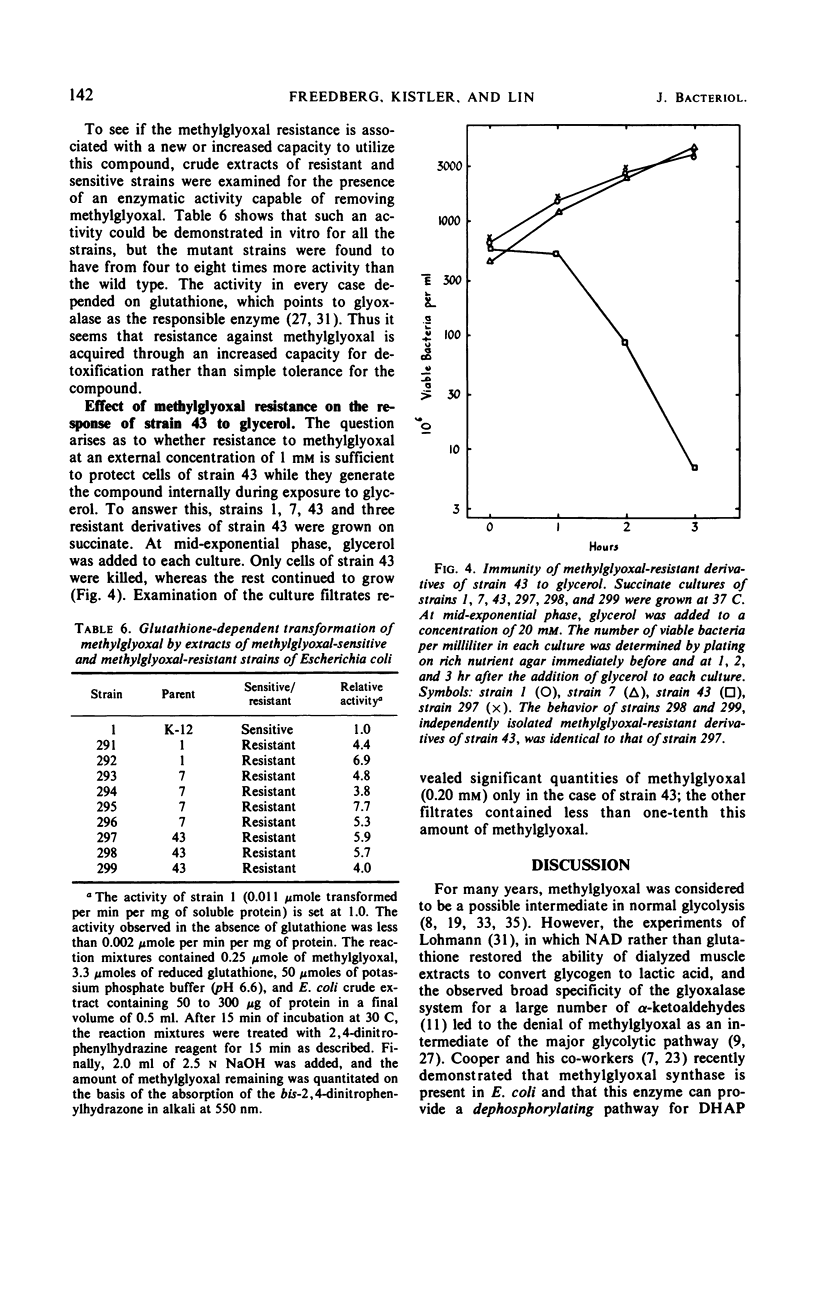
In Escherichia coli K-12, the conversion of glycerol to triose phosphate is regulated by two types of control mechanism: the rate of synthesis of glycerol kinase and the feedback inhibition of its activity by fructose-1,6-diphosphate. A strain which has lost both control mechanisms by successive mutations, resulting in the constitutive synthesis of a glycerol kinase no longer sensitive to feedback inhibition, can produce a bactericidal factor from glycerol. This toxic factor has been identified by chemical and enzymological tests as methylglyoxal. Methylglyoxal can be derived from dihydroxyacetone phosphate through the action of an enzyme which is present at high constitutive levels in the extracts of the mutant as well as that of the wild-type strain. Nine spontaneous mutants resistant to 1 mm exogenous methylglyoxal have been isolated. In all cases the resistance is associated with increased levels of a glutathione-dependent enzymatic activity for the removal of methylglyoxal. Methylglyoxal-resistant mutants derived from the glycerol-sensitive parental strain also became immune to glycerol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A., Cooper R. A. Gluconeogenesis in Escherichia coli The role of triose phosphate isomerase. FEBS Lett. 1969 Jul;4(1):19–20. doi: 10.1016/0014-5793(69)80184-5. [DOI] [PubMed] [Google Scholar]
- Apple M. A., Greenberg D. M. Arrest of cancer in mice by therapy with normal metabolites. II. Indefinite survirors among mice treated with mixtures of 2-oxopropanal (NSC-79019) and 2,3-dihydroxypropanal (NSC67934). Cancer Chemother Rep. 1968 Dec;52(7):687–696. [PubMed] [Google Scholar]
- Berman M., Lin E. C. Glycerol-specific revertants of a phosphoenolpyruvate phosphotransferase mutant: suppression by the desensitization of glycerol kinase to feedback inhibition. J Bacteriol. 1971 Jan;105(1):113–120. doi: 10.1128/jb.105.1.113-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970 Dec 11;11(4):273–276. doi: 10.1016/0014-5793(70)80546-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. H. Aminoacetone formation by Staphylococcus aureus. Biochem J. 1960 Mar;74:478–485. doi: 10.1042/bj0740478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Együd L. G. Studies on cell division: the effect of aldehydes, ketones and alpha-keto-aldehydes on the proliferation of Escherichia coli. Curr Mod Biol. 1967 Mar;1(1):14–20. doi: 10.1016/0303-2647(67)90015-9. [DOI] [PubMed] [Google Scholar]
- Együd L. G., Szent-Györgyi A. Cancerostatic action of methylglyoxal. Science. 1968 Jun 7;160(3832):1140–1140. doi: 10.1126/science.160.3832.1140. [DOI] [PubMed] [Google Scholar]
- Együd L. G., Szent-Györgyi A. Cell division, SH, ketoaldehydes, and cancer. Proc Natl Acad Sci U S A. 1966 Feb;55(2):388–393. doi: 10.1073/pnas.55.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Együd L. G., Szent-Györgyi A. On the regulation of cell division. Proc Natl Acad Sci U S A. 1966 Jul;56(1):203–207. doi: 10.1073/pnas.56.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Turner J. M. Enzymes of methylglyoxal metabolism in a Pseudomonad which rapidly metabolizes aminoacetone. Biochim Biophys Acta. 1969 Jul 30;184(2):464–467. doi: 10.1016/0304-4165(69)90052-x. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Cooper R. A. The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett. 1971 Mar 16;13(4):213–216. doi: 10.1016/0014-5793(71)80538-0. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- KUN E. Inhibition of succinic dehydrogenase by methylglyoxal. J Biol Chem. 1950 Nov;187(1):289–297. [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H., Együd L. G. Locus of the inhibition of protein synthesis by aldo-ketones. Curr Mod Biol. 1968 May-Jun;2(2):106–110. doi: 10.1016/0303-2647(68)90015-4. [DOI] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A., Együd L. G., McLaughlin J. A. Keto-aldehydes and cell division. Science. 1967 Feb 3;155(3762):539–541. doi: 10.1126/science.155.3762.539. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts A. J., Turner J. M. Threonine metabolism in a strain of Bacillus subtilis: enzymes acting on methylglyoxal. Biochim Biophys Acta. 1970 Dec 29;222(3):668–670. doi: 10.1016/0304-4165(70)90195-9. [DOI] [PubMed] [Google Scholar]
- Willetts A. J., Turner J. M. Threonine metabolism in a strain of Bacillus subtilis: enzymic oxidation of the intermediate DL-lactaldehyde. Biochim Biophys Acta. 1970 Oct 27;222(1):234–236. doi: 10.1016/0304-4165(70)90375-2. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Diéguez E. A bactericidal product obtained from a mutant of Escherichia coli. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1415–1422. doi: 10.1016/0006-291x(70)90025-2. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]


