Abstract
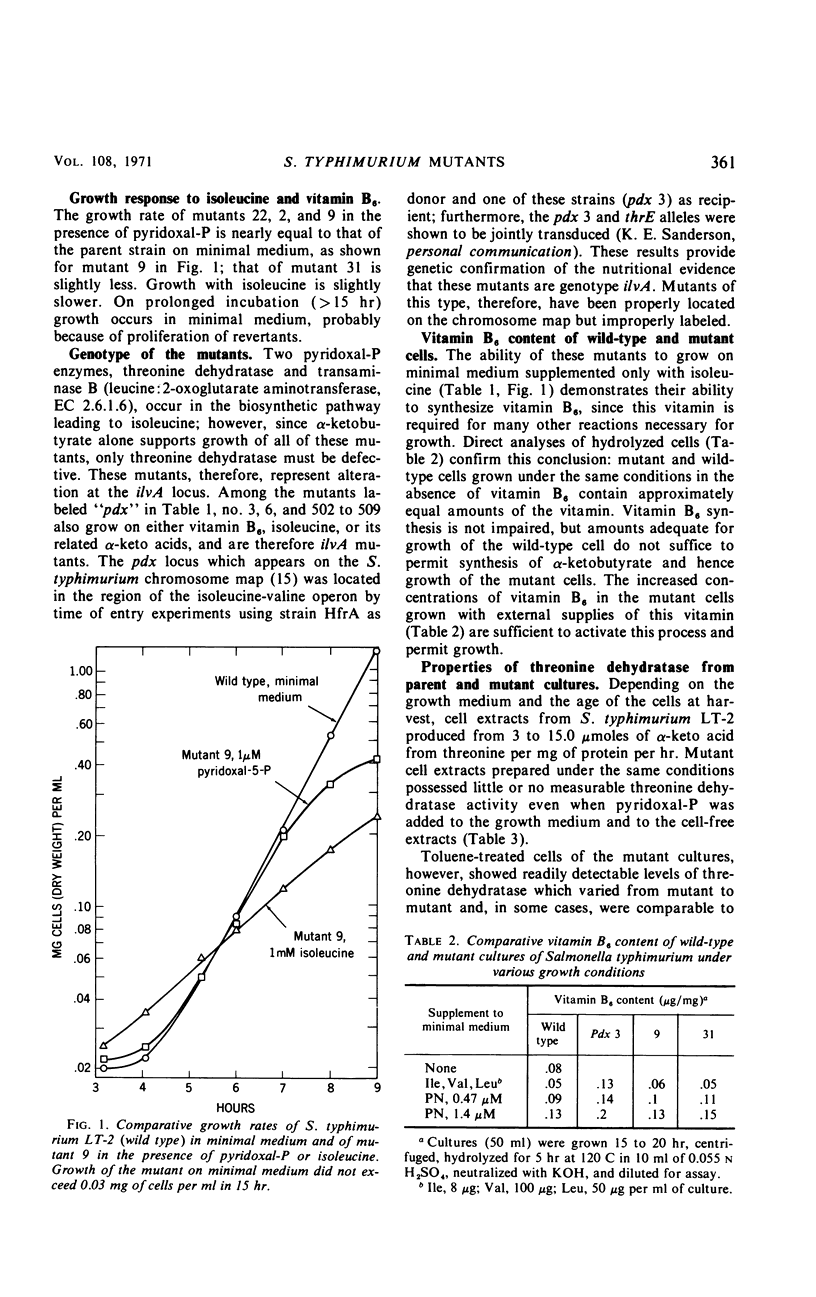
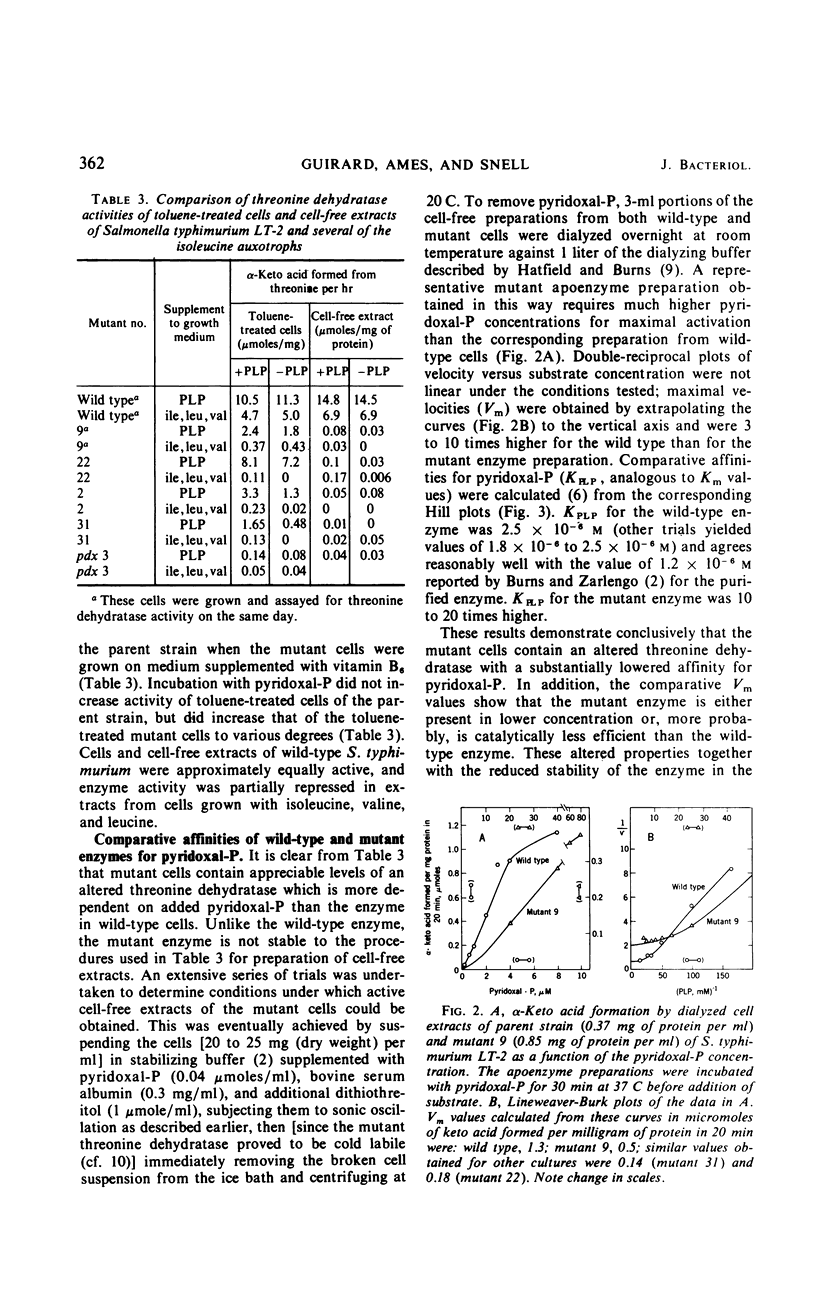
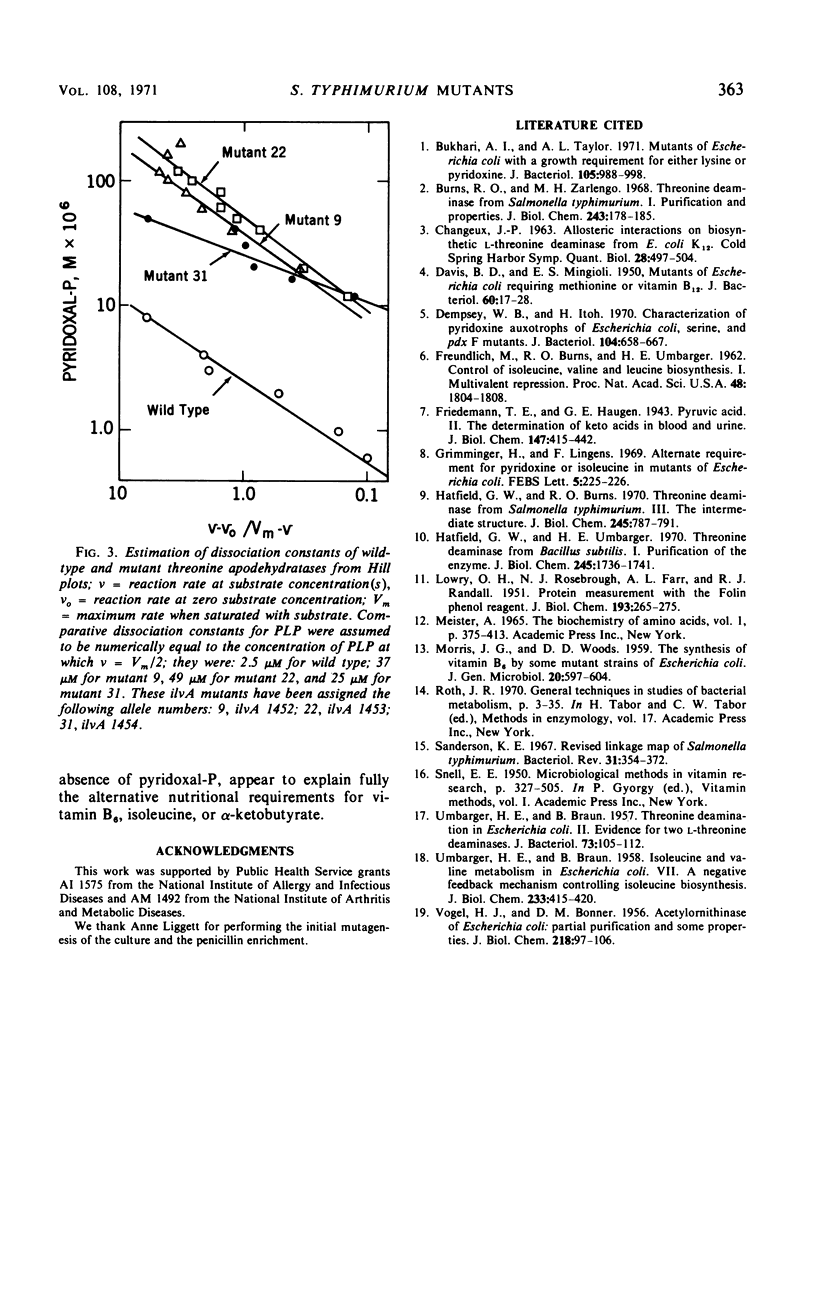
Several mutants of Salmonella typhimurium LT-2, isolated as auxotrophs for vitamin B6, grew without the added vitamin when supplied with either isoleucine, α-ketobutyrate, or α-keto-β-methylvalerate, but not with threonine or with other α-keto acids. When grown on minimal medium supplemented with isoleucine, these mutants synthesized vitamin B6 in amounts comparable to wild-type cells; they thus appeared to contain a modified l-threonine dehydratase and to belong to genotype ilvA (threonine dehydratase) instead of pdx (pyridoxine). Direct assays confirmed this hypothesis. Wild-type cells (toluene-treated) showed approximately the same threonine dehydratase activity whether grown in the presence or absence of added pyridoxal-P; mutant cells approached the activity of wild-type cells only when they were grown with added vitamin B6 and were assayed in the presence of pyridoxal-P. In cell-free extracts, the threonine dehydratase from mutant cells was cold labile and more labile to oxidative inactivation than the wild-type enzyme; furthermore, activation of the mutant apoenzyme required a 10- to 20-fold higher concentration of pyridoxal-P than was required for the wild-type apoenzyme. These results show that cultures which appear auxotrophic for a given vitamin may synthesize that vitamin in normal amounts, the exogenous requirement arising from impaired binding of the vitamin-derived coenzyme to a genetically altered apoenzyme dependent on that coenzyme. Inadequate nutritional data to support the genetic findings can lead to erroneous genotype classification for such mutants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukhari A. I., Taylor A. L. Mutants of Escherichia coli with a growth requirement for either lysine or pyridoxine. J Bacteriol. 1971 Mar;105(3):988–998. doi: 10.1128/jb.105.3.988-998.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Ito H. Characterization of pyridoxine auxotrophs of Escherichia coli: serine and pdxF mutants. J Bacteriol. 1970 Nov;104(2):658–667. doi: 10.1128/jb.104.2.658-667.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Lingens F. Alternate requirement for pyridoxine or isoleucine in mutants of Escherichia coli. FEBS Lett. 1969 Nov 12;5(3):225–226. doi: 10.1016/0014-5793(69)80338-8. [DOI] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Threonine deaminase from Salmonella typhimurium. 3. The intermediate substructure. J Biol Chem. 1970 Feb 25;245(4):787–791. [PubMed] [Google Scholar]
- Hatfield G. W., Umbarger H. E. Threonine deaminase from Bacillus subtilis. I. Purification of the enzyme. J Biol Chem. 1970 Apr 10;245(7):1736–1741. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRIS J. G. The synthesis of vitamin B6 by some mutant strains of Escherichia coli. J Gen Microbiol. 1959 Jun;20(3):597–604. doi: 10.1099/00221287-20-3-597. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VII. A negative feedback mechanism controlling isoleucine biosynthesis. J Biol Chem. 1958 Aug;233(2):415–420. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


