Abstract
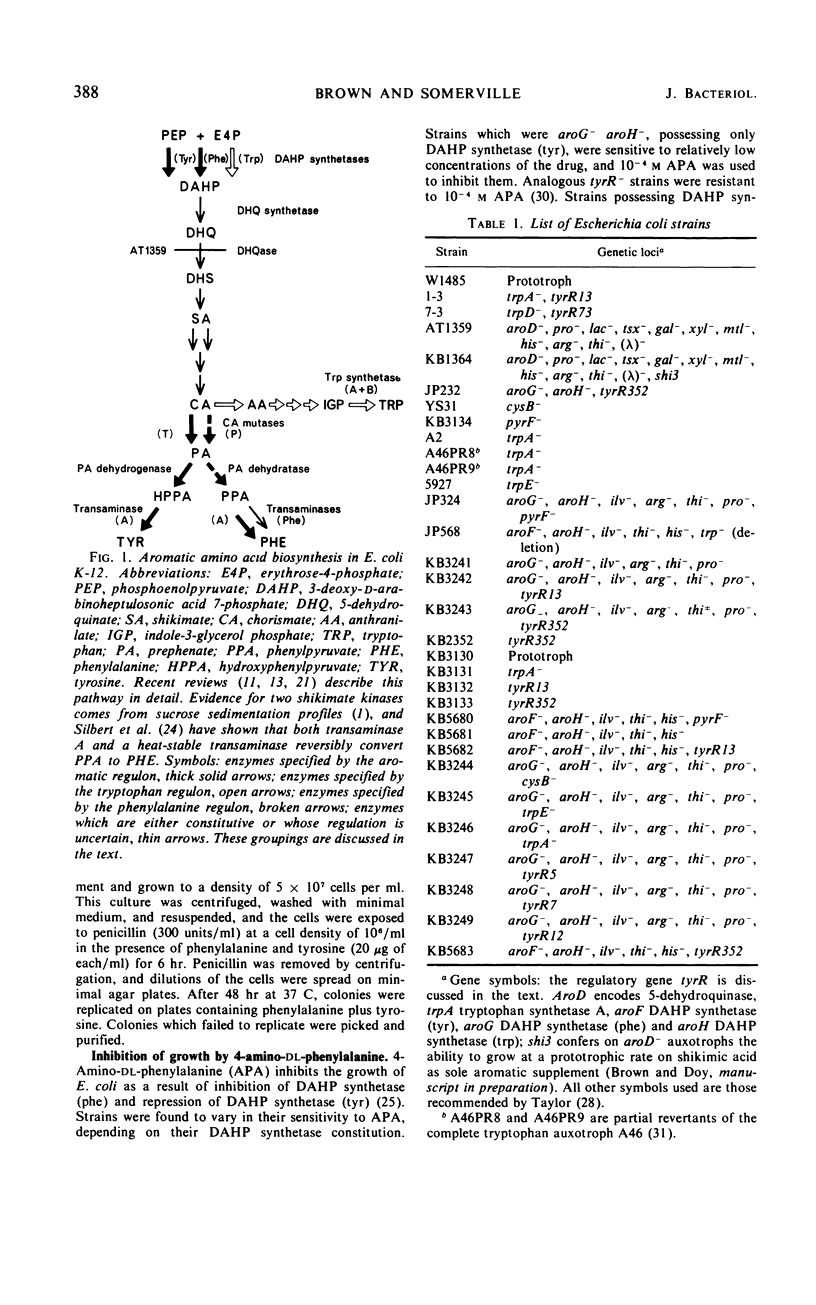
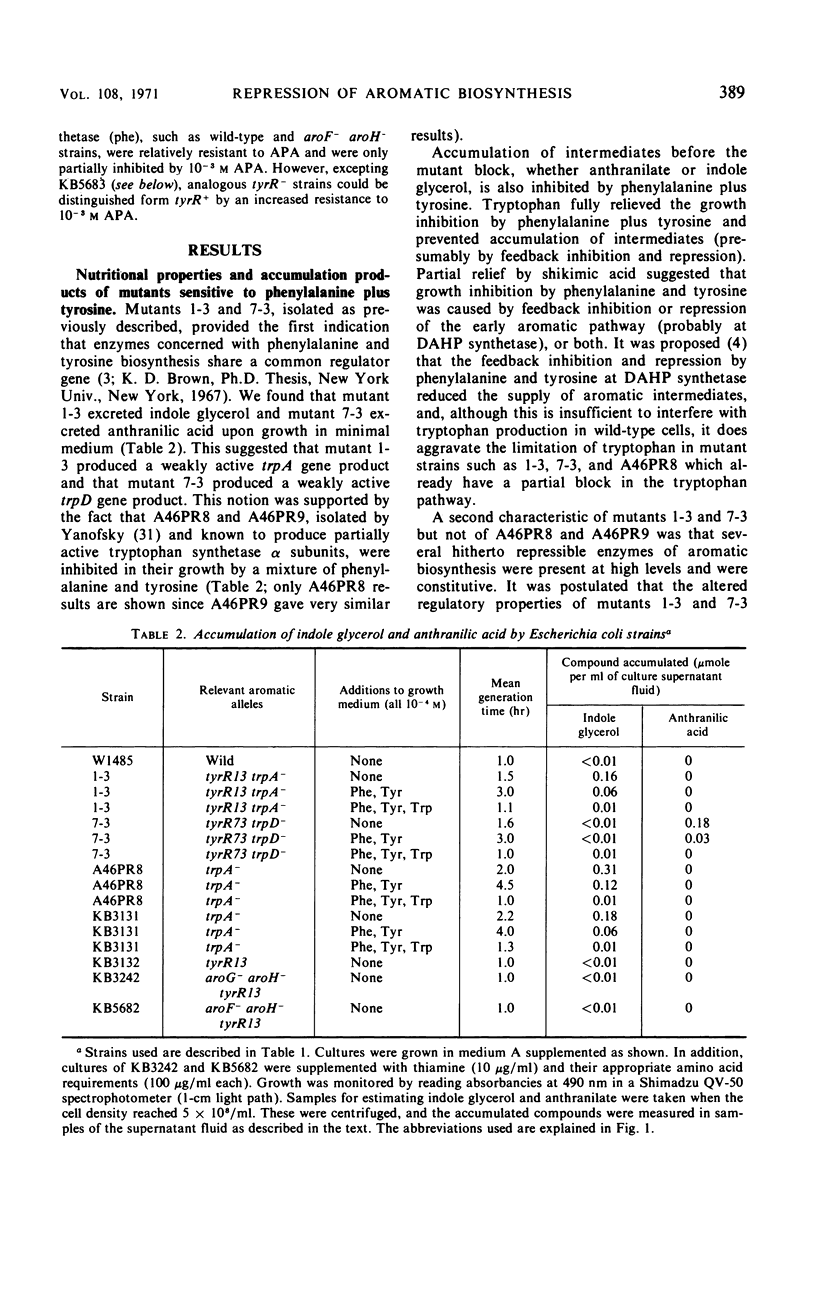
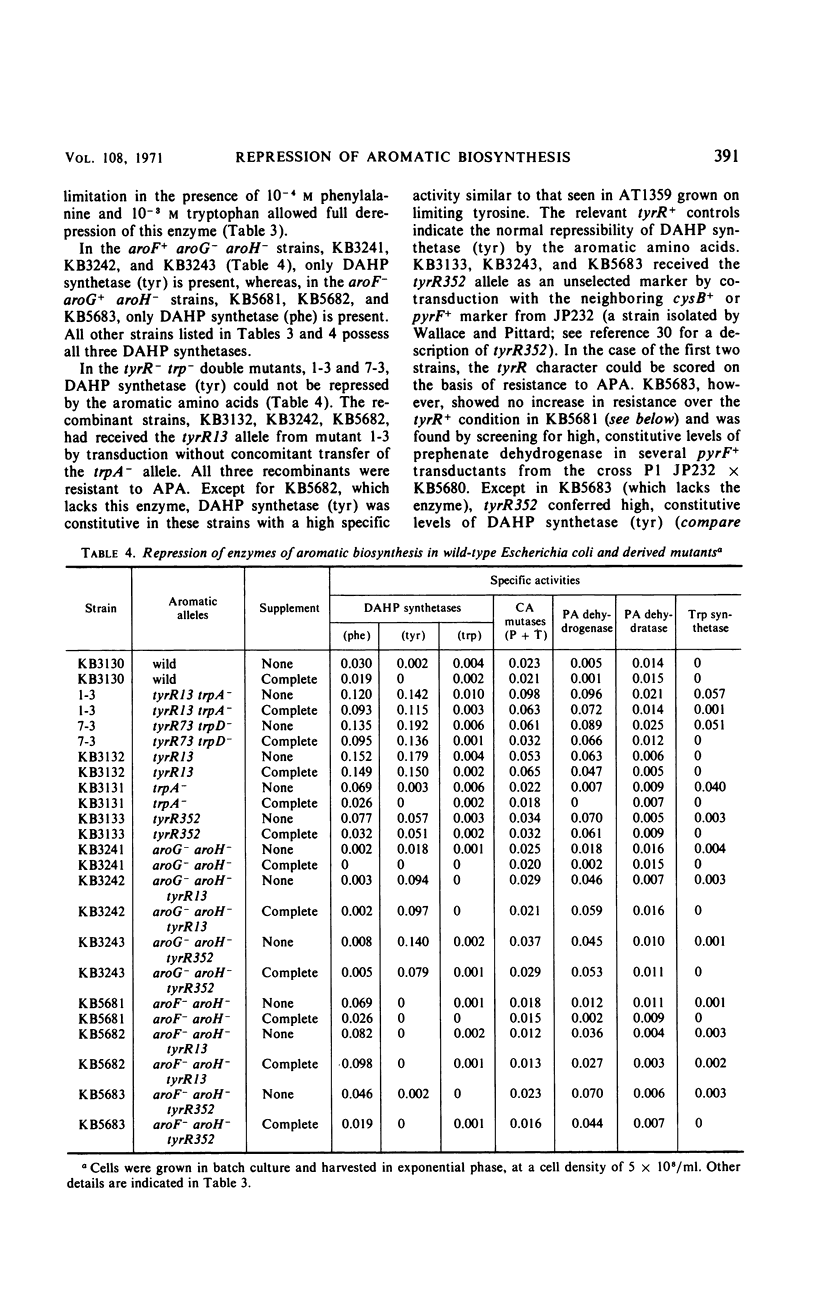
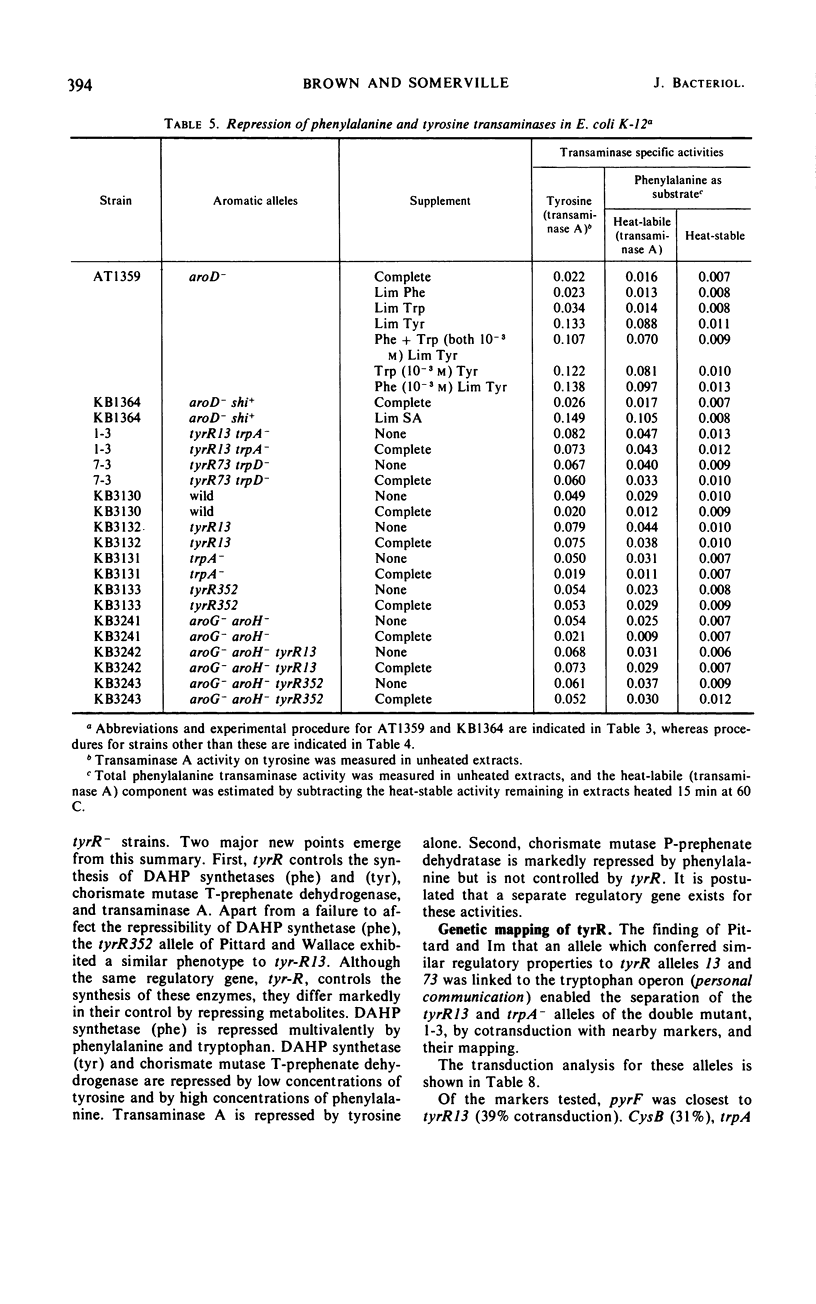
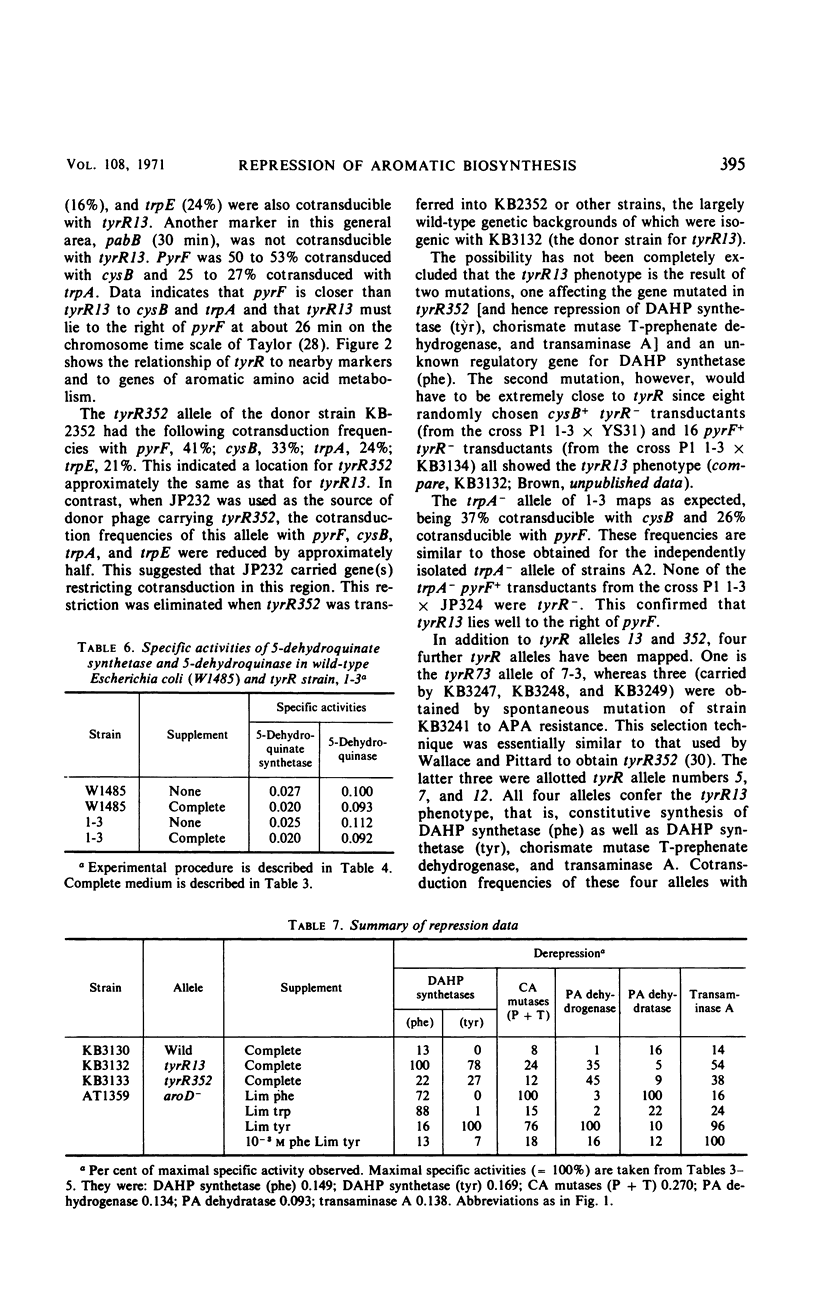
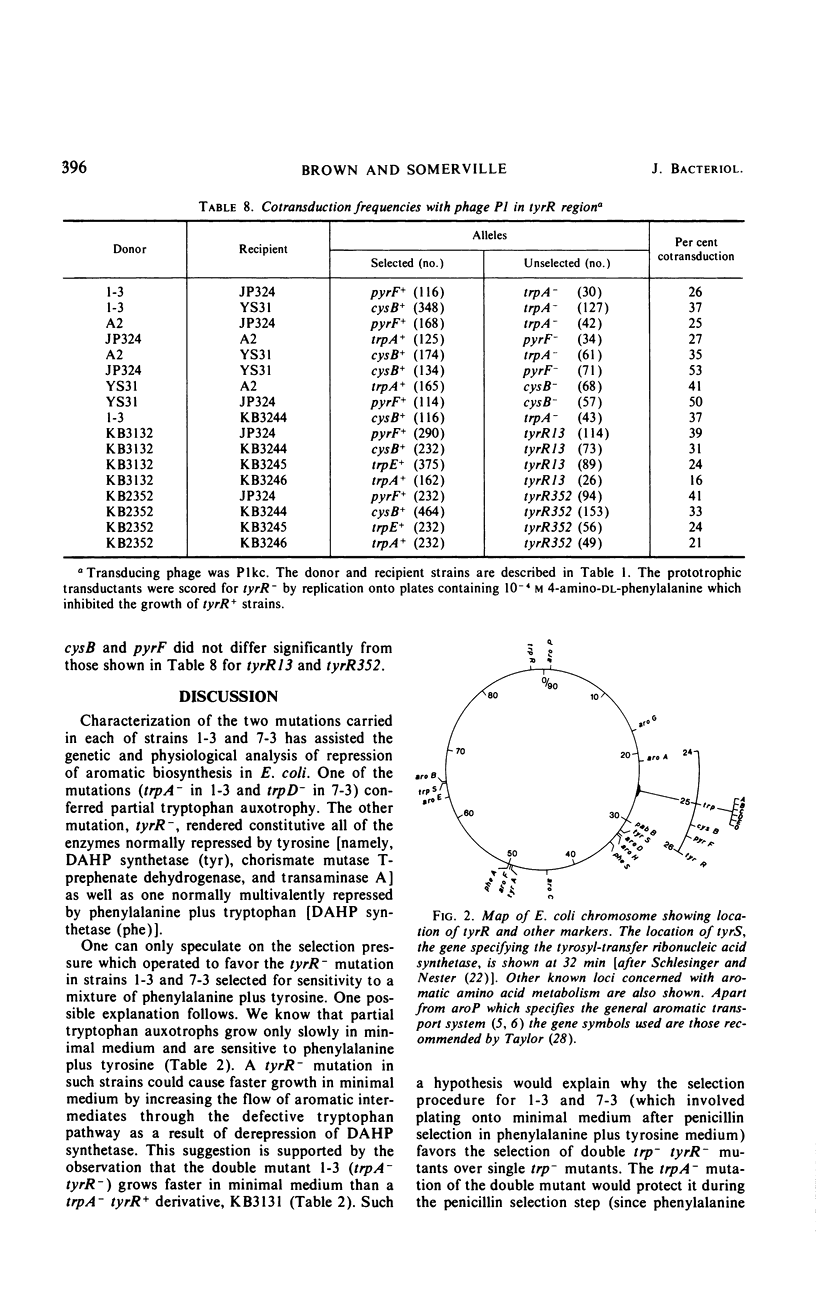
Mutants of Escherichia coli K-12 were isolated in which the synthesis of the following, normally repressible enzymes of aromatic biosynthesis was constitutive: 3-deoxy-d-arabinoheptulosonic acid 7-phosphate (DAHP) synthetases (phe and tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A. In the wild type, DAHP synthetase (phe) was multivalently repressed by phenylalanine plus tryptophan, whereas DAHP synthetase (tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A were repressed by tyrosine. DAHP synthetase (tyr) and chorismate mutase T-prephenate dehydrogenase were also repressed by phenylalanine in high concentration (10−3m). Besides the constitutive synthesis of DAHP synthetase (phe), the mutants had the same phenotype as strains mutated in the tyrosine regulatory gene tyrR. The mutations causing this phenotype were cotransducible with trpA, trpE, cysB, and pyrF and mapped in the same region as tyrR at approximately 26 min on the chromosome. It is concluded that these mutations may be alleles of the tyrR gene and that synthesis of the enzymes listed above is controlled by this gene. Chorismate mutase P and prephenate dehydratase activities which are carried on a single protein were repressed by phenylalanine alone and were not controlled by tyrR. Formation of this protein is presumed to be controlled by a separate, unknown regulator gene. The heat-stable phenylalanine transaminase and two enzymes of the common aromatic pathway, 5-dehydroquinate synthetase and 5-dehydroquinase, were not repressible under the conditions studied and were not affected by tyrR. DAHP synthetase (trp) and tryptophan synthetase were repressed by tryptophan and have previously been shown to be under the control of the trpR regulatory gene. These enzymes also were unaffected by tyrR.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlyn M. B., Giles N. H. Organization of enzymes in the polyaromatic synthetic pathway: separability in bacteria. J Bacteriol. 1969 Jul;99(1):222–230. doi: 10.1128/jb.99.1.222-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Doy C. H. Control of three isoenzymic 7-phospho-2-oxo-3-deoxy-Darabino-heptonate-D-erythrose-4-phosphate lyases of Escherichia coli W and derived mutants by repressive and "inductive" effects of the aromatic amino acids. Biochim Biophys Acta. 1966 Apr 12;118(1):157–172. doi: 10.1016/s0926-6593(66)80153-4. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Maintenance and exchange of the aromatic amino acid pool in Escherichia coli. J Bacteriol. 1971 Apr;106(1):70–81. doi: 10.1128/jb.106.1.70-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON F., YANOFSKY C. The partial purification and properties of indole-3-glycerol phosphate synthetase from Escherichia coli. Biochim Biophys Acta. 1960 Oct 7;43:489–500. doi: 10.1016/0006-3002(60)90471-6. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Davidson H., Pittard J. Phenylalanine and tyrosine biosynthesis in Escherichia coli K-12: mutants derepressed for 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (phe), 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A. J Bacteriol. 1971 Oct;108(1):400–409. doi: 10.1128/jb.108.1.400-409.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Pittard J., Camakaris J., Wallace B. J. Inhibition of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (trp) in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1242–1247. doi: 10.1128/jb.97.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- SILBERT D. F., JORGENSEN S. E., LIN E. C. Repression of transaminase A by tyrosine in Escherichia coli. Biochim Biophys Acta. 1963 Jun 11;73:232–240. doi: 10.1016/0006-3002(63)90307-x. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. THE EFFECTS OF PHENYLALANINE AND TYROSINE ANALOGS ON THE SYNTHESIS AND ACTIVITY OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE SYNTHETASES. Arch Biochem Biophys. 1964 May;105:424–430. doi: 10.1016/0003-9861(64)90026-8. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. Some aspects of amino acid biosynthesis in microorganisms. Annu Rev Biochem. 1968;37:79–108. doi: 10.1146/annurev.bi.37.070168.000455. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


