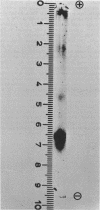
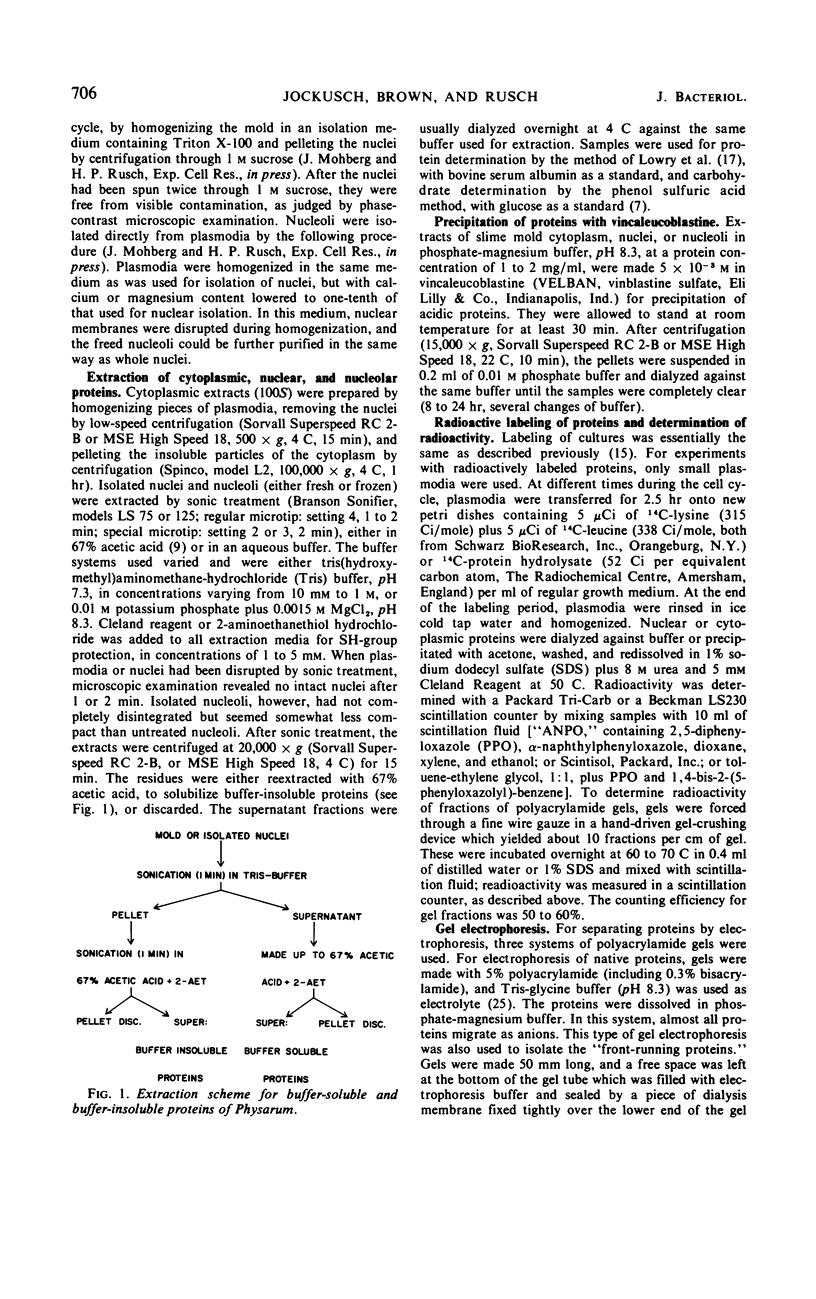
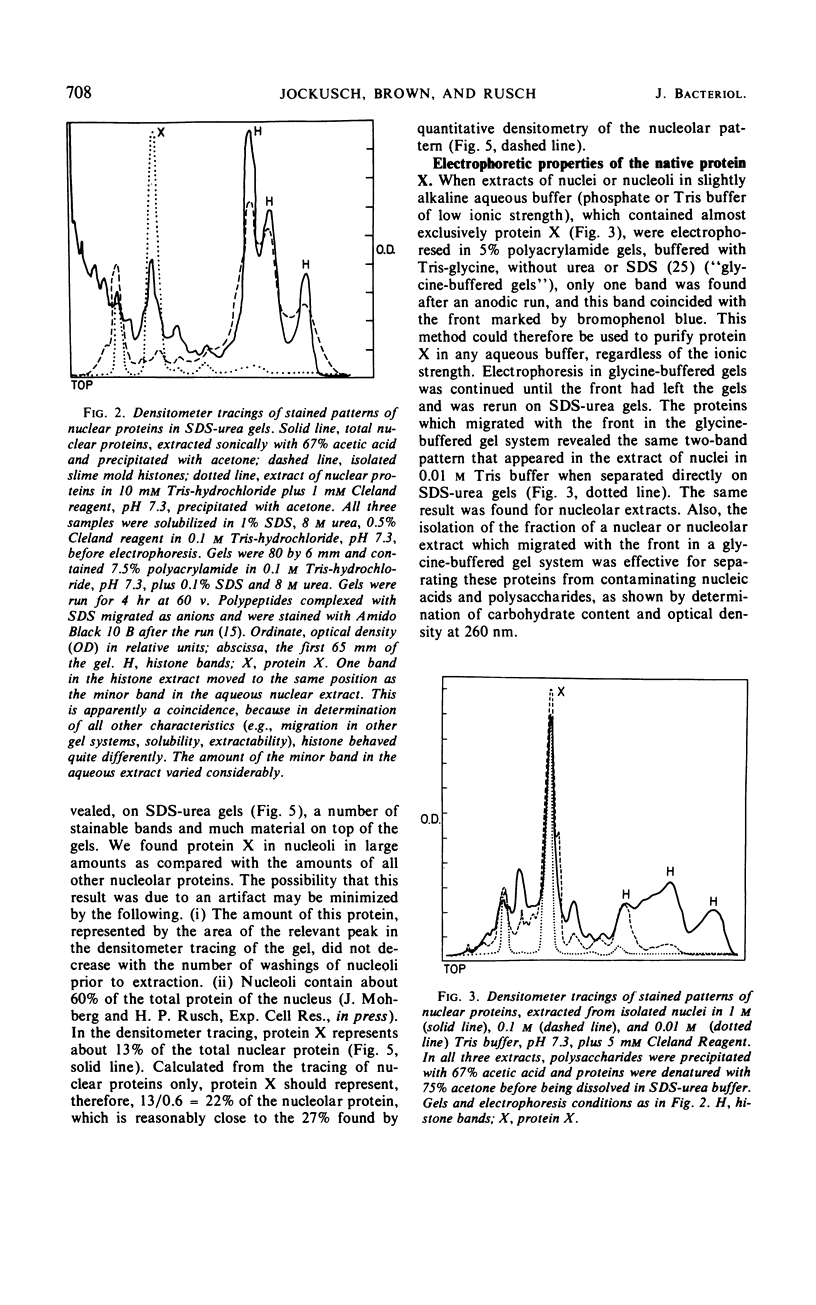
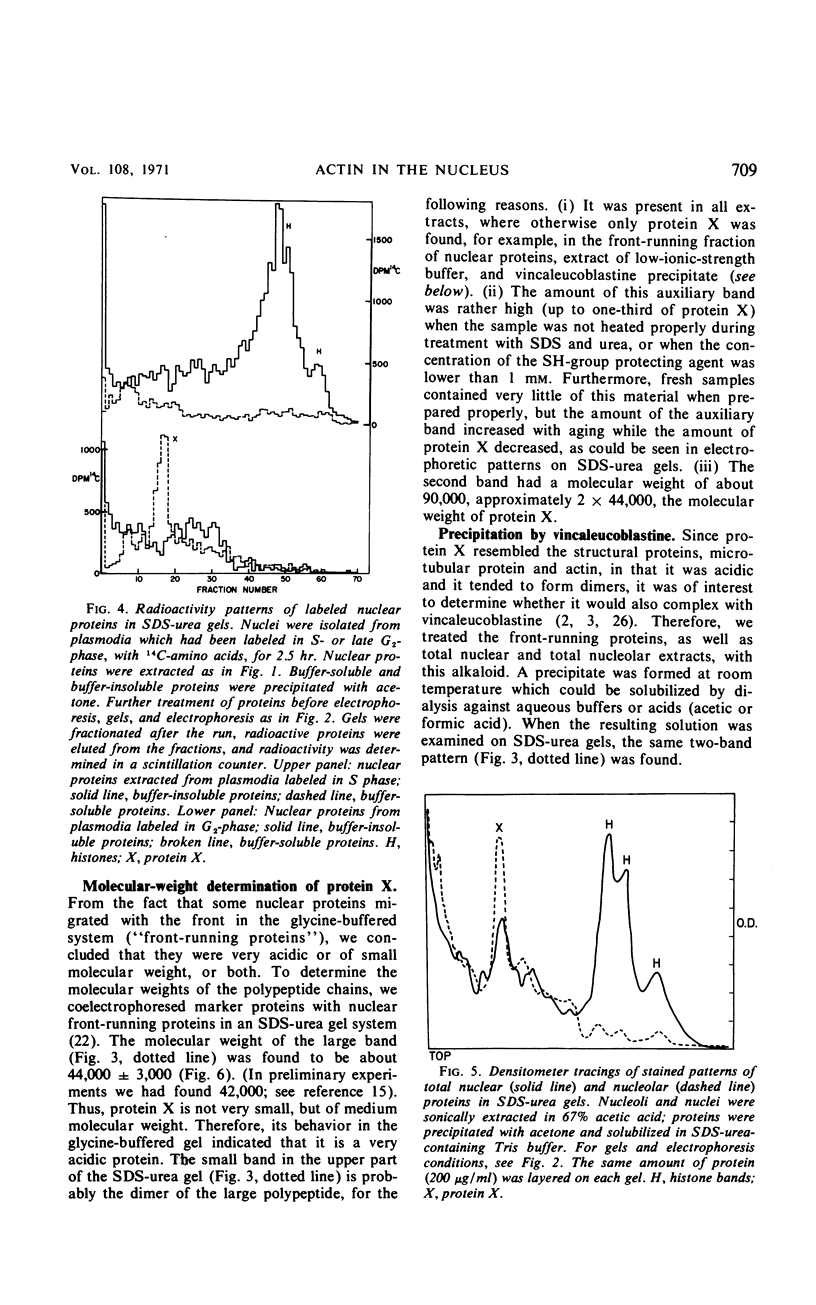
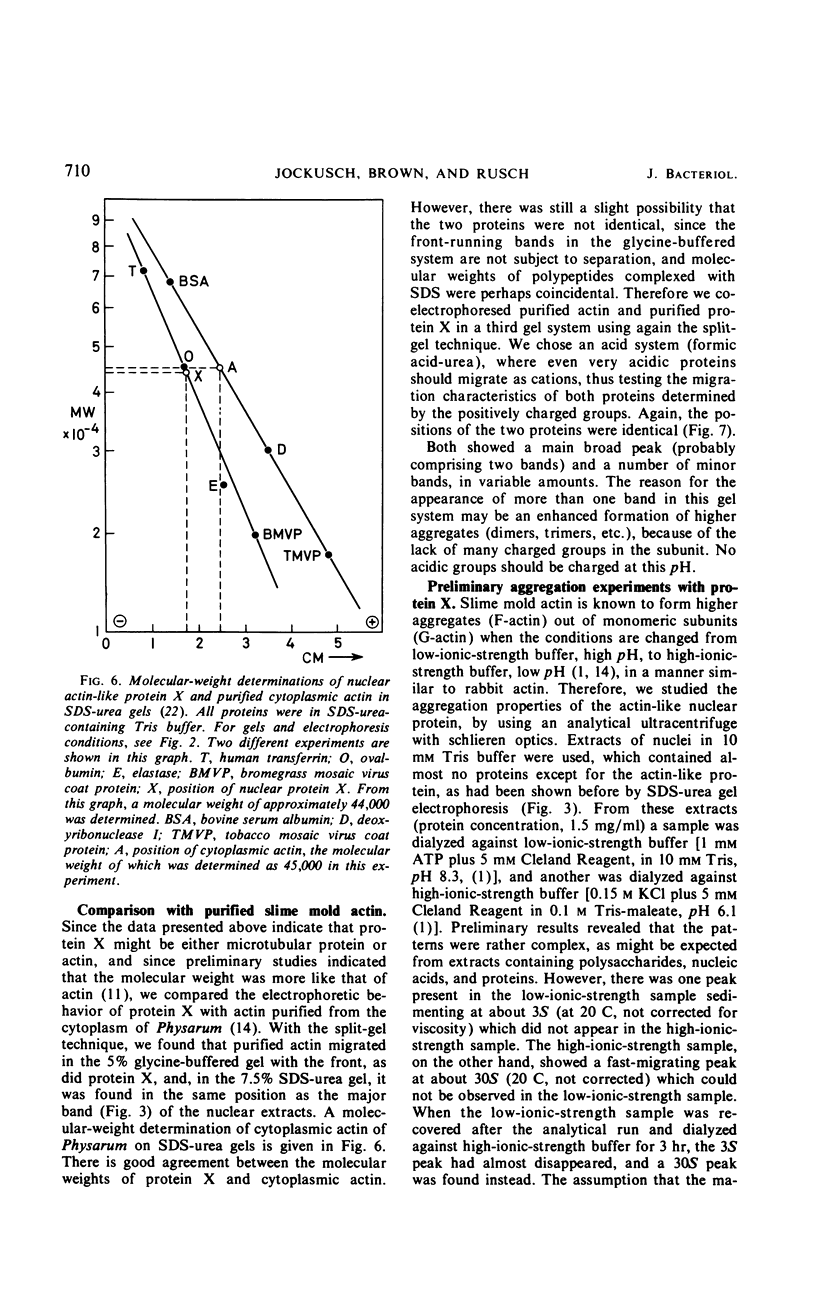
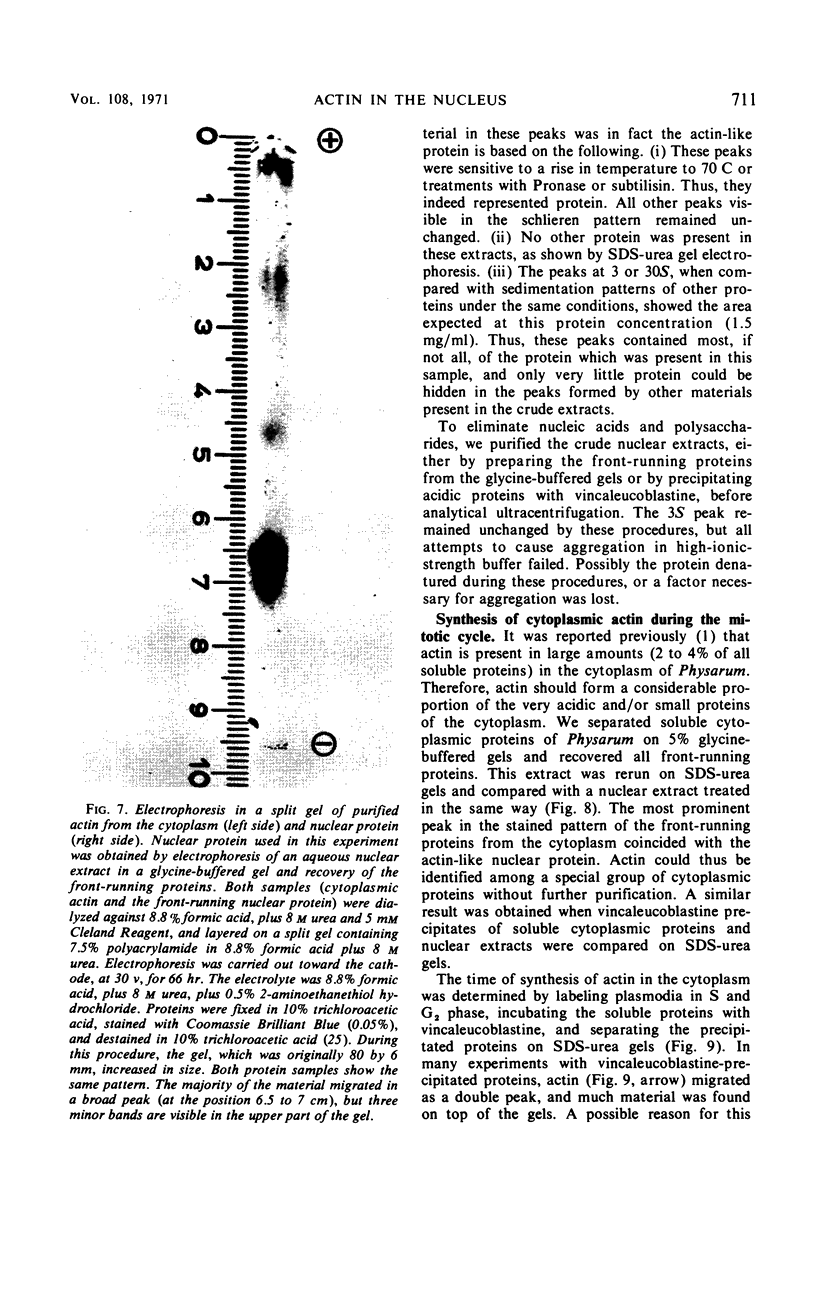
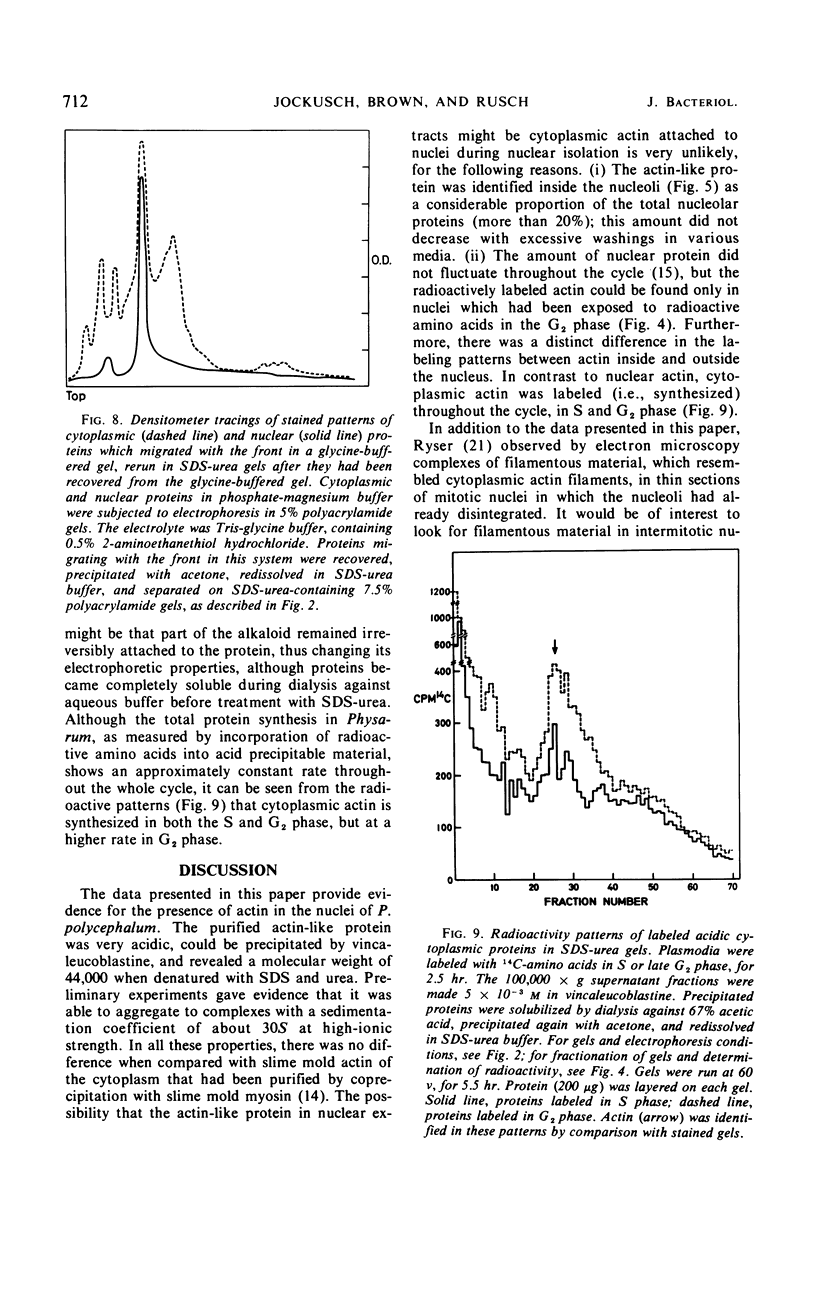
Abstract
A protein was extracted from isolated nuclei of the slime mold Physarum polycephalum which could be labeled with radioactive precursors only during G2 phase. The native protein was purified by extraction in low-ionic-strength buffer [10 mm tris(hydroxymethyl)aminomethane-hydrochloride] of isolated nuclei and by preparative polyacrylamide gel electrophoresis. It was extracted from isolated nucleoli. Its electrophoretic properties in three different polyacrylamide gel systems, its molecular weight (44,000 ± 3,000), its precipitability by vincaleucoblastine, a vinca alkaloid, and its aggregation properties suggested that it might be actin. In a direct comparison with slime mold actin purified from the cytoplasm, no difference could be found between the two proteins in all these characteristics. The synthesis of cytoplasmic actin was not found to occur exclusively during G2 phase. This suggested that nuclear actin was either synthesized independently from cytoplasmic actin or transported into the nuclei exclusively during G2 phase. The possible role of nuclear actin during intranuclear mitosis is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Taylor E. W. Further purification and characterization of slime mold myosin and slime mold actin. Biochemistry. 1969 Dec;8(12):4976–4988. doi: 10.1021/bi00840a047. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Malawista S. E. Microtubule crystals: a new biophysical phenomenon induced by Vinca alkaloids. Nature. 1968 Jun 22;218(5147):1176–1177. doi: 10.1038/2181176a0. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Marantz R., Wisniewski H., Shelanski M. Induction in vitro of microtubular crystals by vinca alkaloids. Science. 1969 Aug 1;165(3892):495–496. doi: 10.1126/science.165.3892.495. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957 Aug;4(1):1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- Forer A. Characterization of the mitotic traction system, and evidence that birefringent spindle fibers neither produce nor transmit force for chromosome movement. Chromosoma. 1966;19(1):44–98. doi: 10.1007/BF00332793. [DOI] [PubMed] [Google Scholar]
- GUTTES E., GUTTES S., RUSCH H. P. Morphological observations on growth and differentation of Physarum polycephalum grown in pure culture. Dev Biol. 1961 Oct;3:588–614. doi: 10.1016/0012-1606(61)90034-3. [DOI] [PubMed] [Google Scholar]
- Gosselin-Rey C., Gerday C., Gaspar-Godfroid A., Carsten M. E. Amino acid analysis and peptide mapping of bovine carotid actin. Biochim Biophys Acta. 1969 Feb 4;175(1):165–173. doi: 10.1016/0005-2795(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Guttes S., Guttes E., Ellis R. A. Electron microscope study of mitosis in Physarum polycephalum. J Ultrastruct Res. 1968 Mar;22(5):508–529. doi: 10.1016/s0022-5320(68)90038-5. [DOI] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Isolation and characterization of plasmodium actin. Biochim Biophys Acta. 1966 Oct 31;127(2):488–498. doi: 10.1016/0304-4165(66)90402-8. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Brown D. F., Rusch H. P. Synthesis of a nuclear protein in G2-phase. Biochem Biophys Res Commun. 1970 Jan 23;38(2):279–283. doi: 10.1016/0006-291x(70)90709-6. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Sauer H. W., Brown D. F., Babcock K. L., Rusch H. P. Differential protein synthesis during sporulation in the slime mold Physarum polycephalum. J Bacteriol. 1970 Aug;103(2):356–363. doi: 10.1128/jb.103.2.356-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Growth of large plasmodia of the myxomycete Physarum polycephalum. J Bacteriol. 1969 Mar;97(3):1411–1418. doi: 10.1128/jb.97.3.1411-1418.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Nuclear histones in Physarum polycephalum during growth and differentiation. Arch Biochem Biophys. 1970 Jun;138(2):418–432. doi: 10.1016/0003-9861(70)90365-6. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser U. Die Ultrastruktur der Mitosekerne in den Plasmodien von Physarum polycephalum. Z Zellforsch Mikrosk Anat. 1970;110(1):108–130. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. On the apparent homology of actin and tubulin. Science. 1970 May 15;168(3933):845–847. doi: 10.1126/science.168.3933.845. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Reassociation of microtubule protein. J Mol Biol. 1968 Apr 28;33(2):517–519. doi: 10.1016/0022-2836(68)90210-6. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]