Abstract
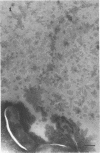
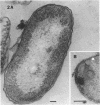
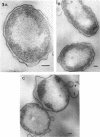
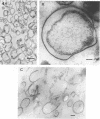
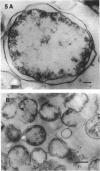
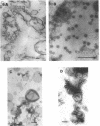
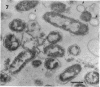
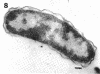
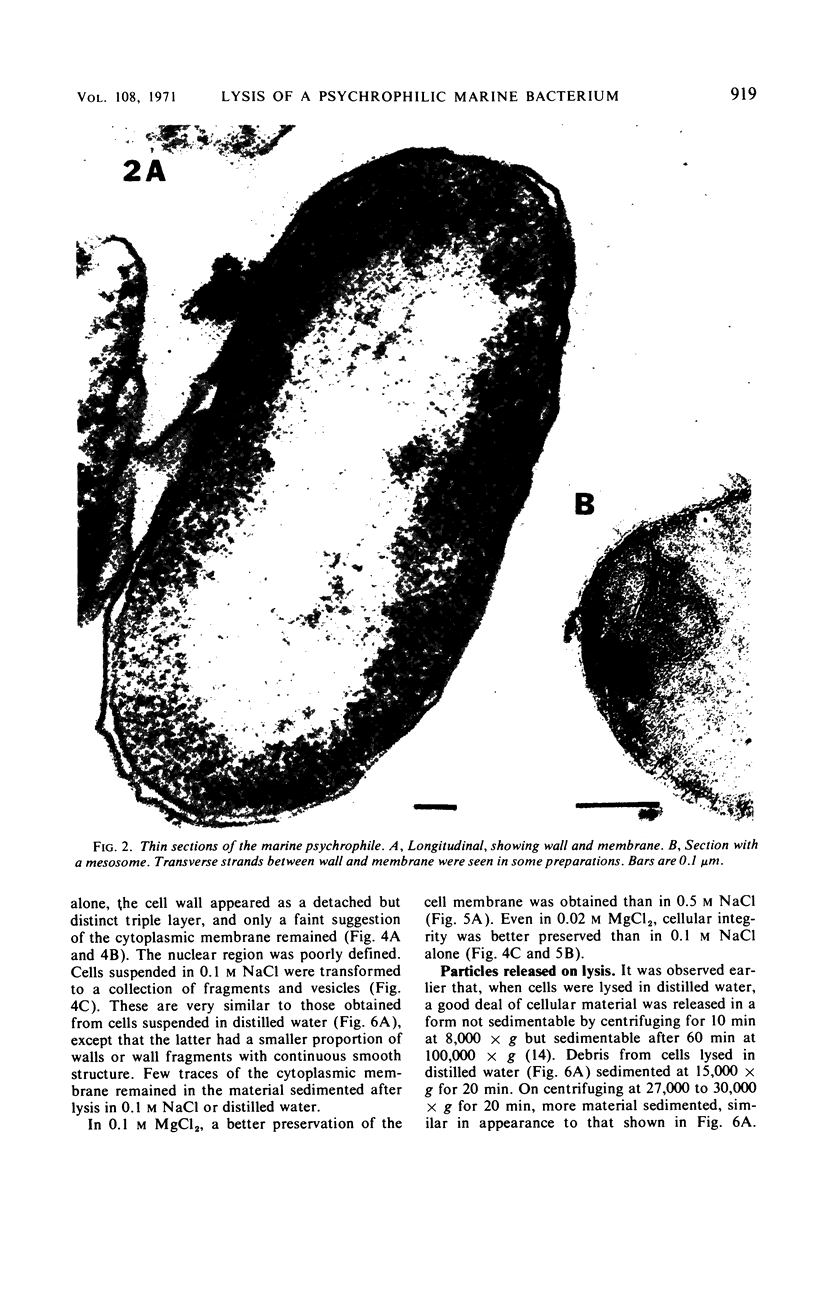
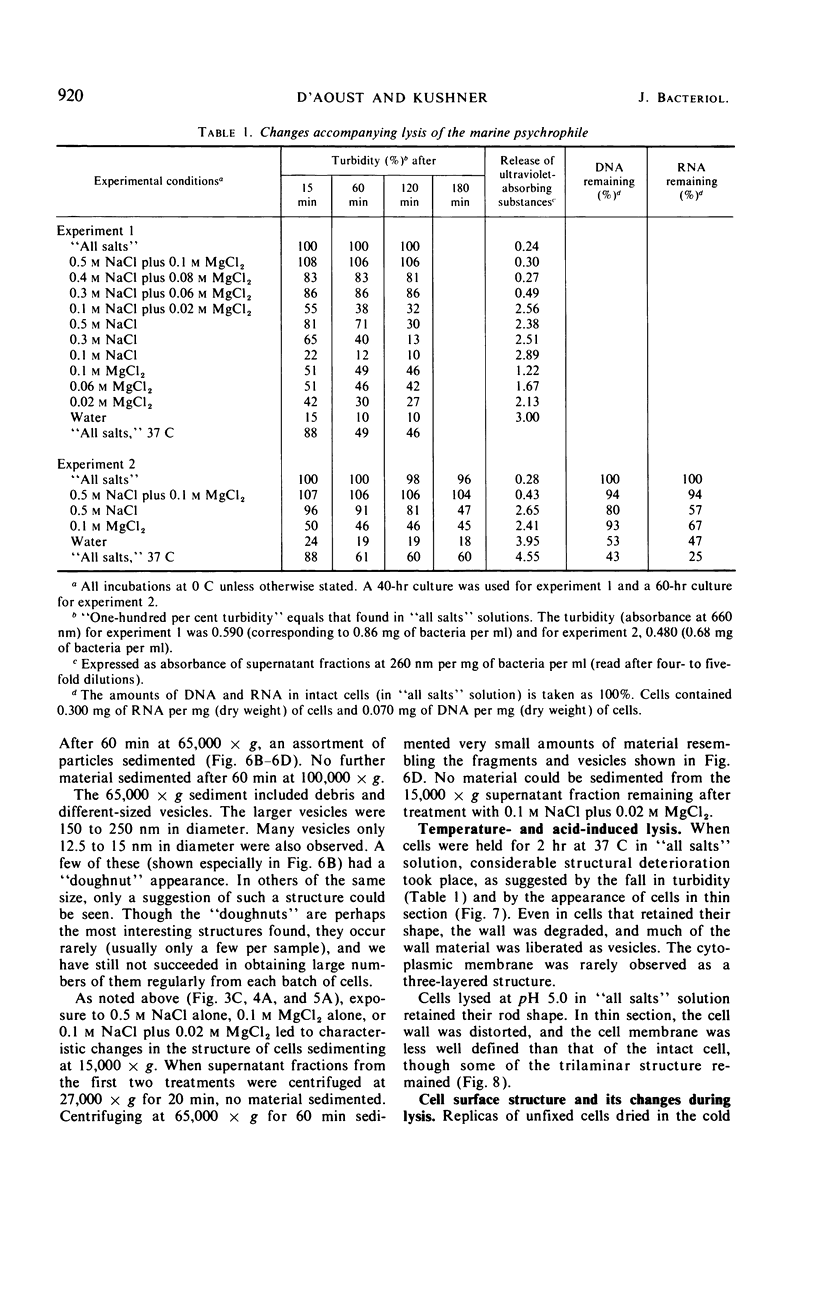
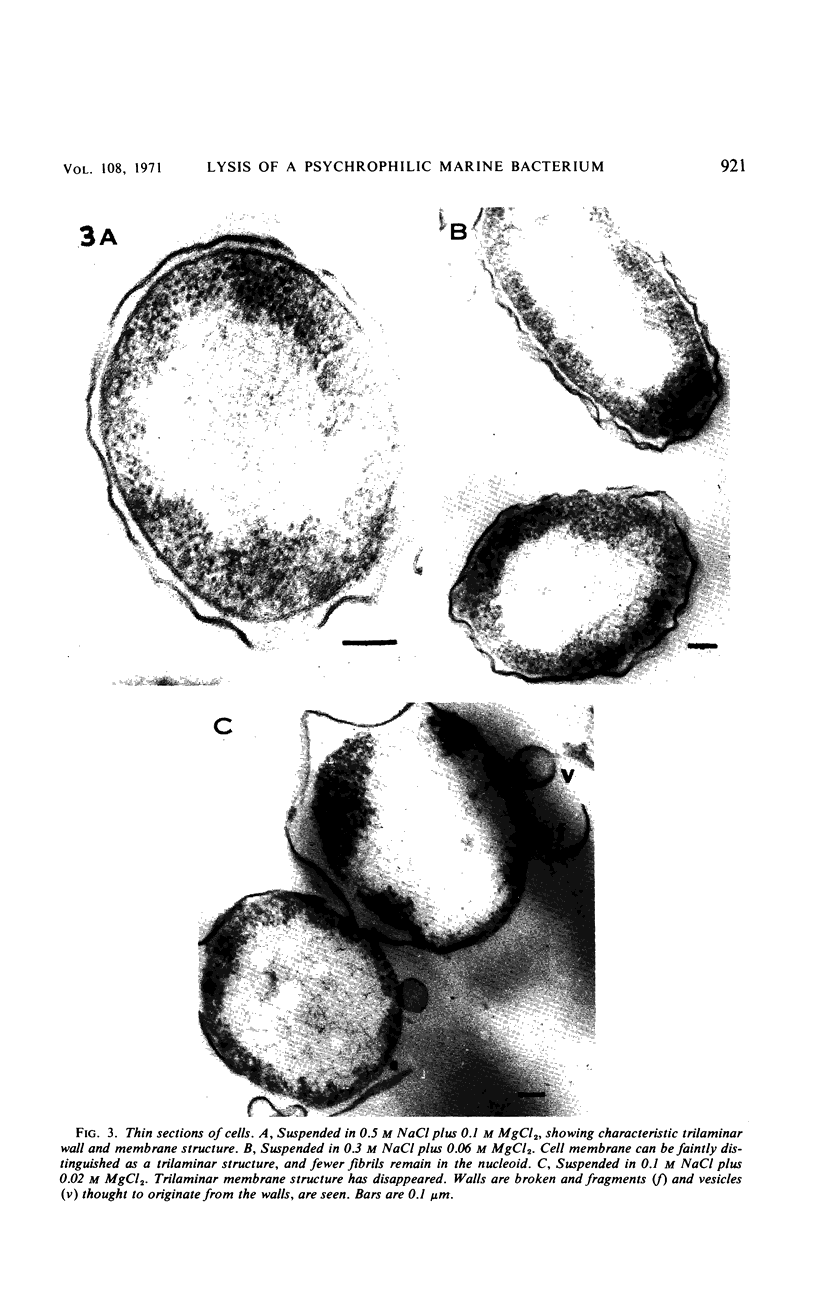
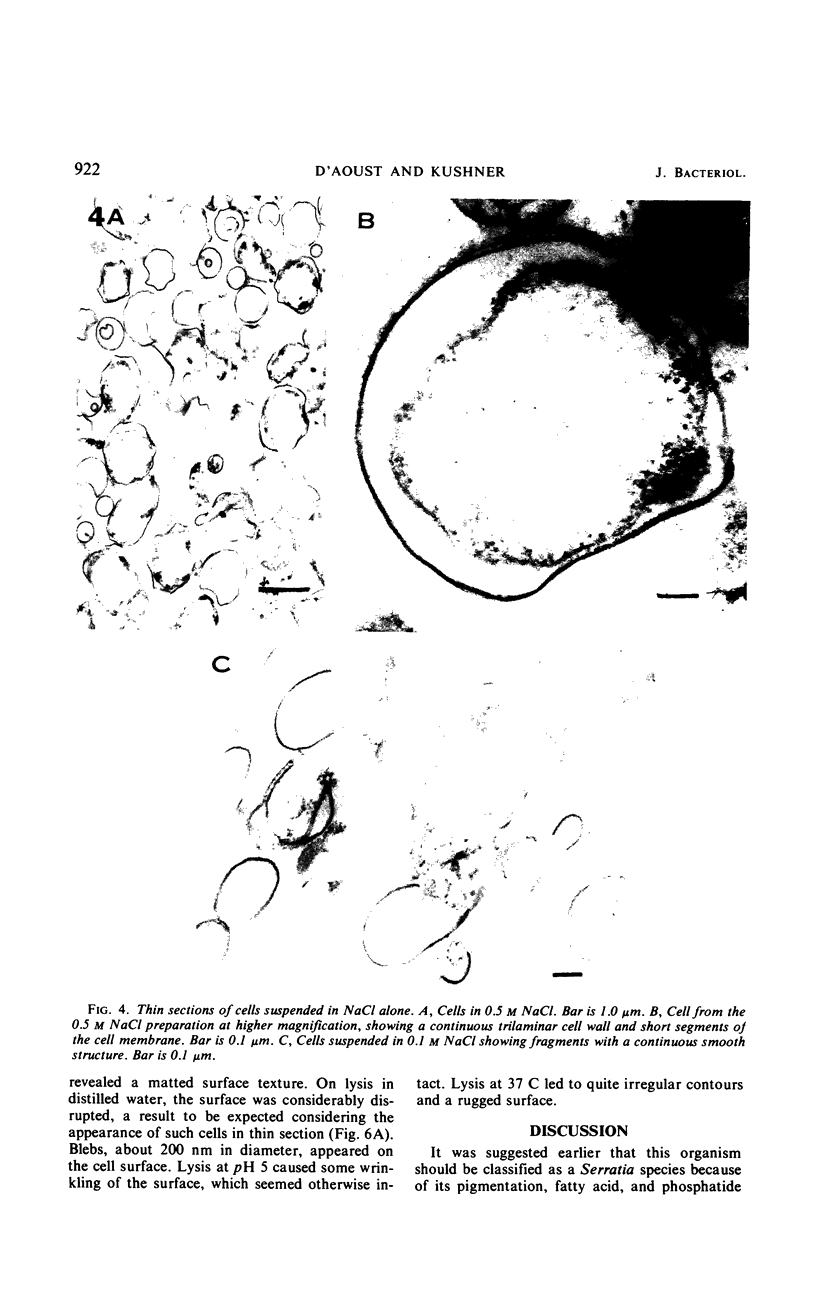
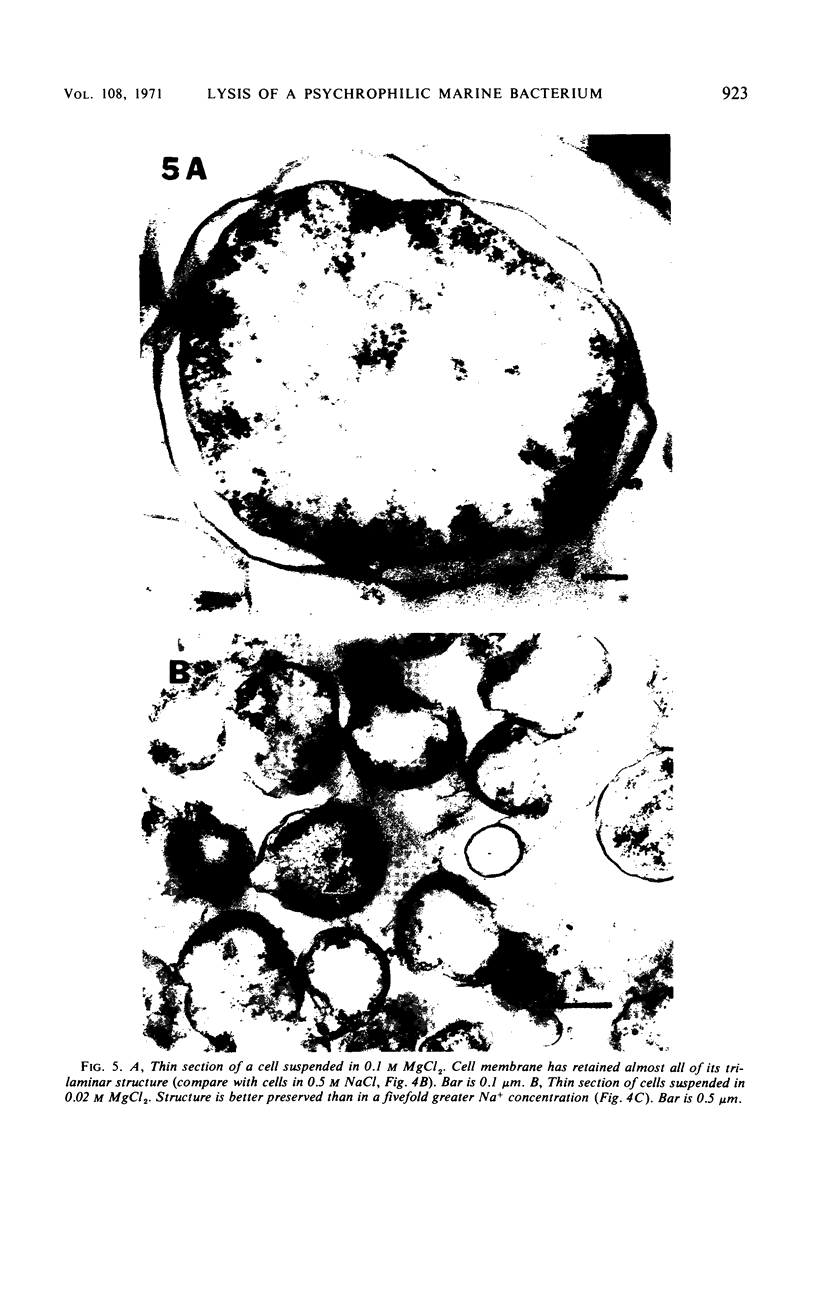
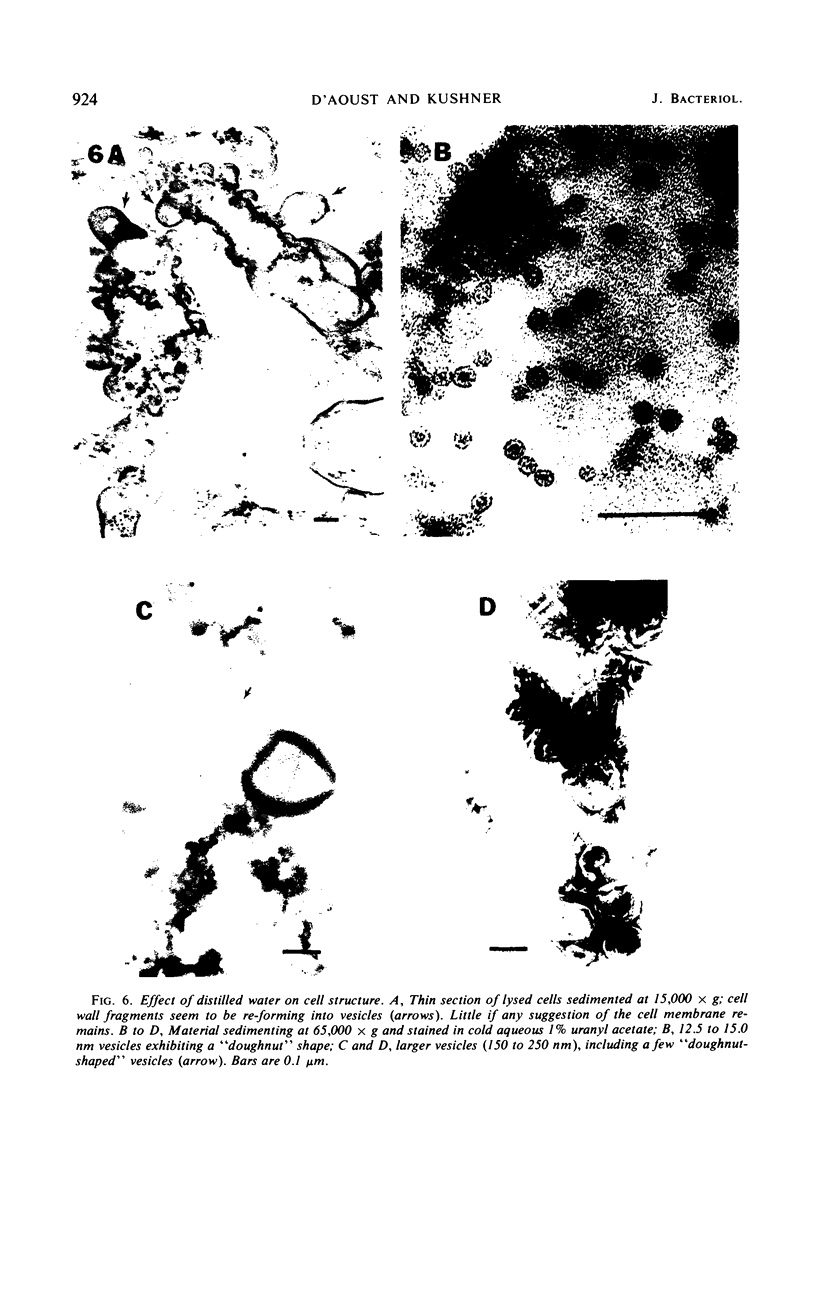
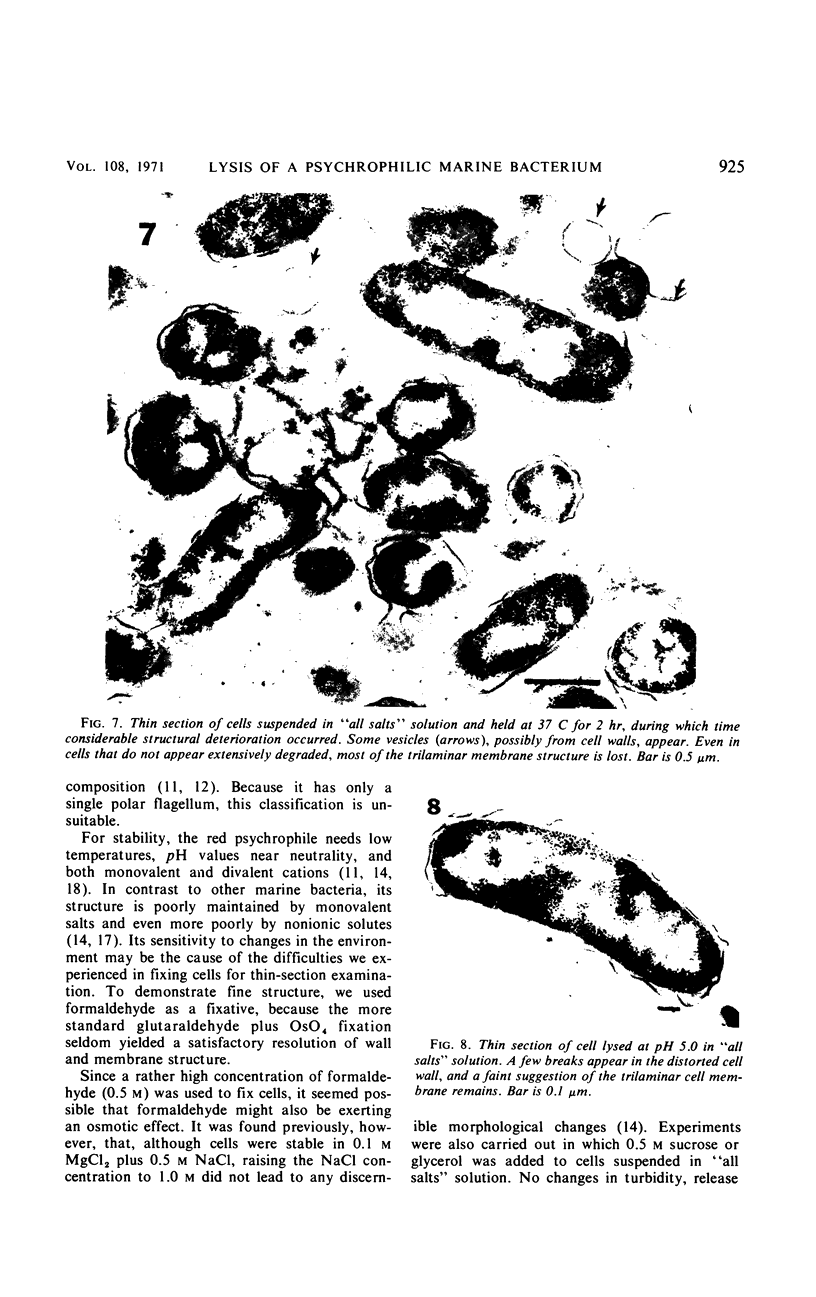
The marine psychrophile, a red, gram-negative motile rod with a single polar flagellum, is stable when suspended in 0.1 m Mg2+ plus 0.5 m NaCl at 0 C and neutral pH but lyses if the salt composition of the medium is changed, the temperature raised above 20 C, or the pH lowered. Lysis is accompanied by a fall in turbidity, a release of ultraviolet-absorbing substances, and a loss of deoxyribonucleic acid and ribonucleic acid. Ultrastructural changes accompanying lysis were studied. Thin sections of cells fixed while intact showed a triple-layered cell wall and cytoplasmic membrane, each 6.0 to 7.5 nm thick. Mesosomes were also observed. Either Na+ or Mg2+ could maintain wall integrity, whereas Mg2+ was needed for membrane integrity. In distilled water, lysis was very extensive, and much material was released as wall fragments and as vesicles which probably came from the wall and cytoplasmic membrane. Lysis at 37 C resulted in degradation of the wall and liberation of wall fragments. The cell membrane was rarely observed as a triple-layered structure in such temperature-lysed cells. After lysis at pH 5.0, the cell wall was distorted, and only a suggestion of the cell membrane remained. Replicas showed that this organism had a matted surface which was distorted under different conditions of lysis.
Full text
PDF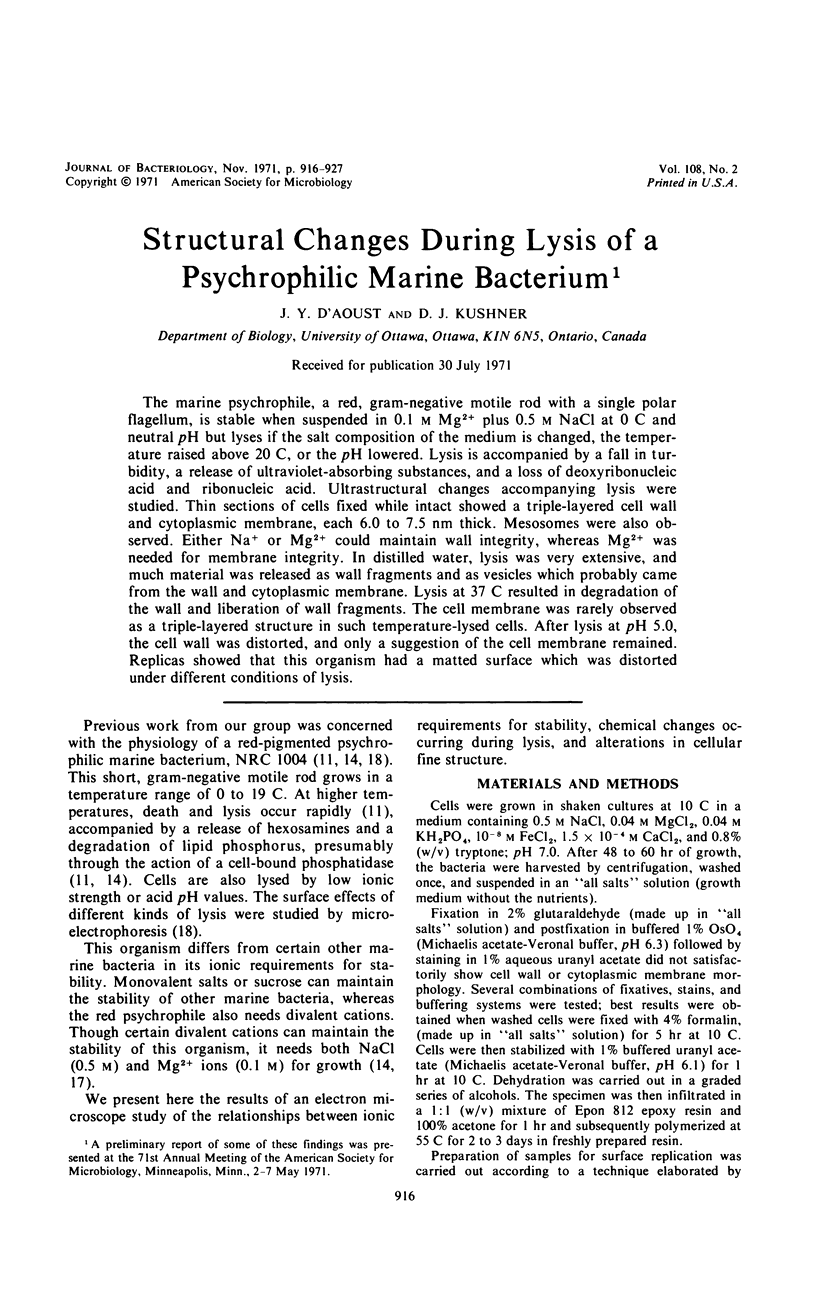




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961 Oct;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- ABRAM D., GIBBONS N. E. Turbidity of suspensions and morphology of red halophilic bacteria as influenced by sodium chloride concentration. Can J Microbiol. 1960 Oct;6:535–543. doi: 10.1139/m60-062. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. THE PERIPHERAL STRUCTURES OF GRAM-NEGATIVE BACTERIA.IV. THE CATION-SENSITIVE DISSOLUTION OF THE CELL MEMBRANE OF THE HALOPHILIC BACTERIUM, HALOBACTERIUM HALOBIUM. Biochim Biophys Acta. 1963 Nov 29;75:425–435. doi: 10.1016/0006-3002(63)90630-9. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Fleischer B., Stoeckenius W. Fine structure of lipid-depleted mitochondria. J Cell Biol. 1967 Jan;32(1):193–208. doi: 10.1083/jcb.32.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- HAGEN P. O., KUSHNER D. J., GIBBONS N. E. TEMPERATURE-INDUCED DEATH AND LYSIS IN A PSYCHROPHILIC BACTERIUM. Can J Microbiol. 1964 Dec;10:813–822. doi: 10.1139/m64-106. [DOI] [PubMed] [Google Scholar]
- KATES M., HAGEN P. O. INFLUENCE OF TEMPERATURE ON FATTY ACID COMPOSITION OF PSYCHROPHILIC AND MESOPHILIC SERRATIA SPECIES. Can J Biochem. 1964 Apr;42:481–488. doi: 10.1139/o64-055. [DOI] [PubMed] [Google Scholar]
- KUSHNER D. J., BAYLEY S. T., BORING J., KATES M., GIBBONS N. E. MORPHOLOGICAL AND CHEMICAL PROPERTIES OF CELL ENVELOPES OF THE EXTREME HALOPHILE, HALOBACTERIUM CUTIRUBRUM. Can J Microbiol. 1964 Jun;10:483–497. doi: 10.1139/m64-058. [DOI] [PubMed] [Google Scholar]
- Korngold R. R., Kushner D. J. Responses of a psychrophilic marine bacterium to changes in its ionic environment. Can J Microbiol. 1968 Mar;14(3):253–263. doi: 10.1139/m68-042. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]