Abstract
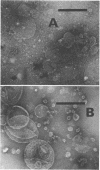
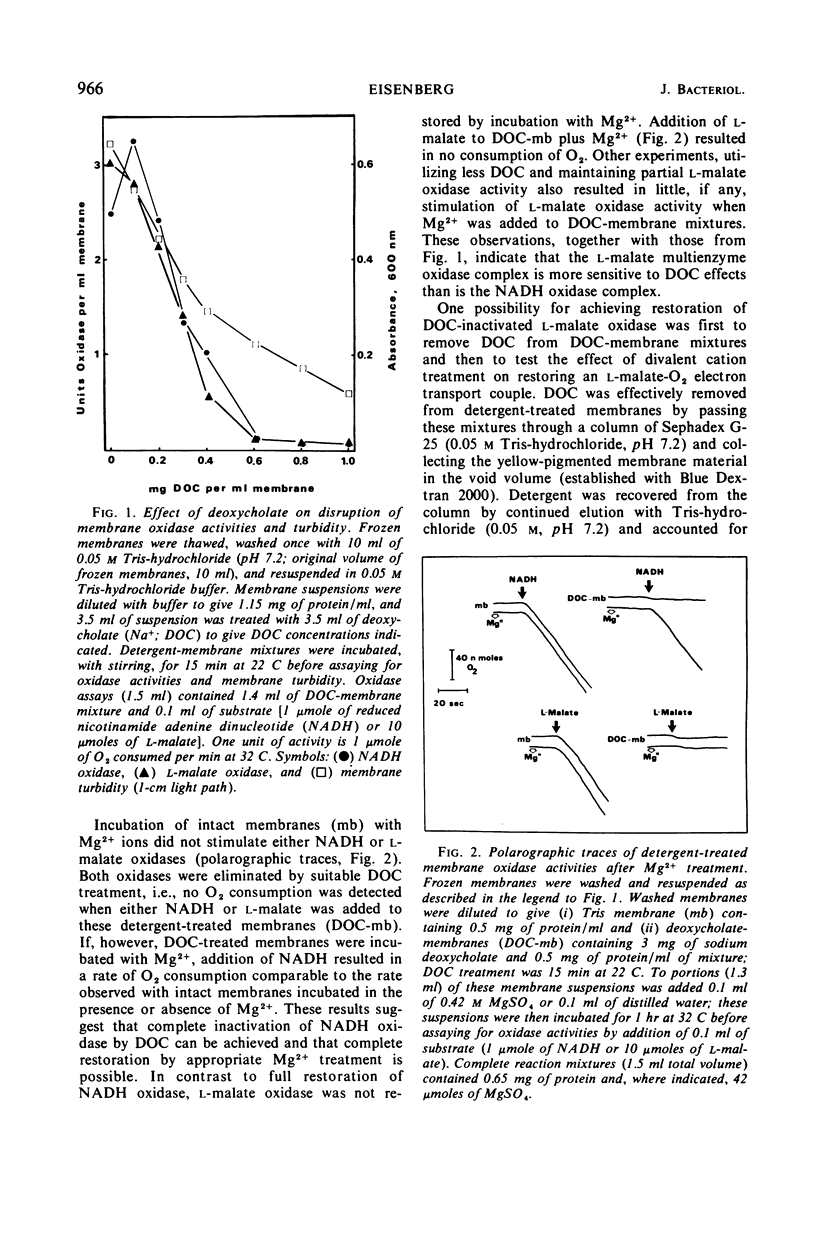
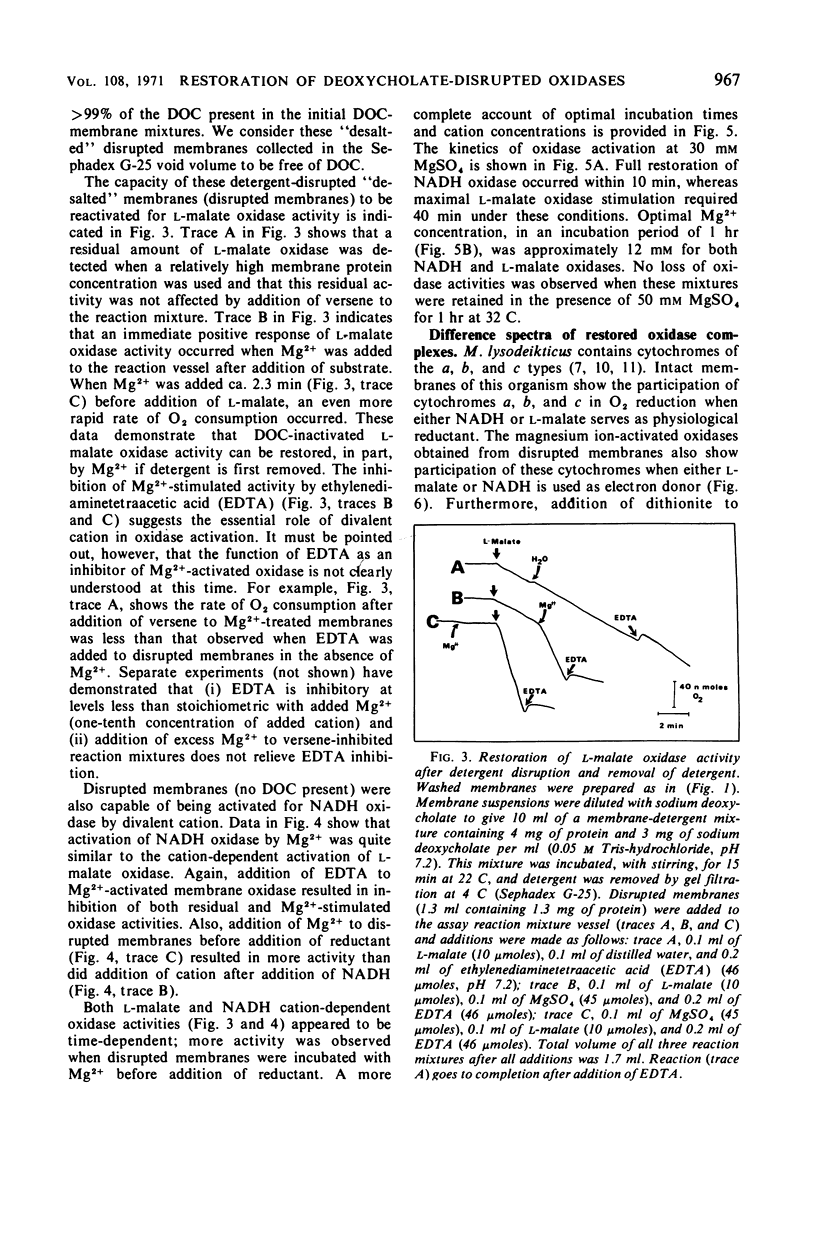
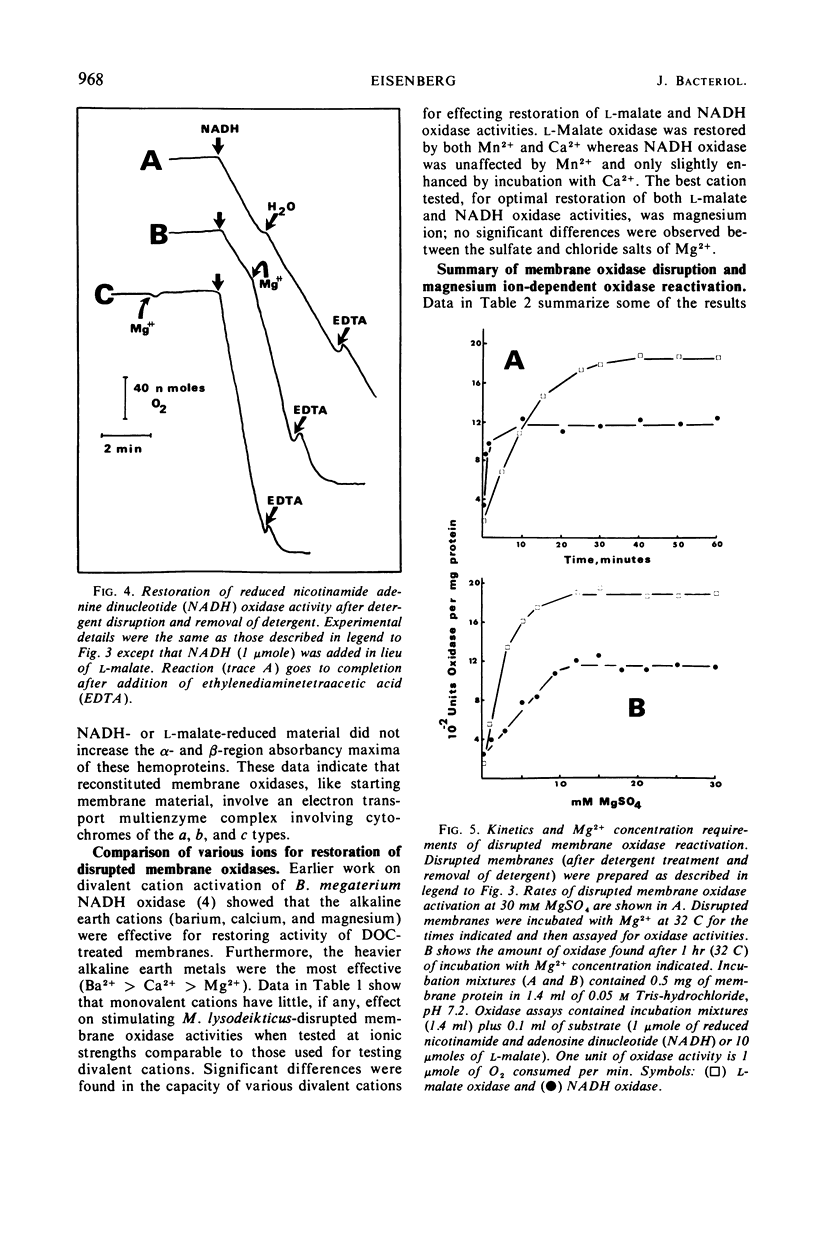
Membrane-associated l-malate and reduced nicotinamide adenine dinucleotide (NADH) oxidase complexes of Micrococcus lysodeikticus were inactivated with deoxycholate. Reactivation of NADH oxidase by addition of Mg2+ occurred in these detergent-membrane mixtures, but reactivation of l-malate oxidase did not occur in the presence of deoxycholate. Removal of detergent by gel filtration allowed Mg2+-dependent restoration of both l-malate and NADH oxidases. Maximal NADH and l-malate oxidase restoration required 10 min and 40 min, respectively, at 30 mm MgSO4. Maximal restoration of both oxidases required at least 12 mm MgSO4 in an incubation period of 1 hr. Reduced-minus-oxidized difference spectra of Mg2+-restored membrane oxidases showed participation of cytochromes b, c, and a when either l-malate or NADH served as reductant; addition of dithionite did not increase the α- and β-region absorbancy maxima of these hemoproteins when restored membranes were first reduced with the physiological substrates l-malate or NADH. Not all divalent cations tested were equally effective for reactivation of both oxidases. l-Malate oxidase was restored by both Mn2+ and Ca2+. NADH oxidase was not activated by Mn2+ and only slightly stimulated by Ca2+. Separation of deoxycholate-disrupted membranes (detergent removed) into soluble and particulate fractions showed that both fractions were required for Mg2+-dependent oxidase activities. Electron micrographs indicated conditions of detergent treatment did not destroy the vesicular nature of protoplast ghost membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN D. V. The enzymatic formation of oxalacetic acid by nonpyridine nucleotide malic dehydrogenase of Micrococcus lysodeikticus. J Biol Chem. 1958 Aug;233(2):299–304. [PubMed] [Google Scholar]
- COHN D. V. The oxidation of malic acid by Micrococcus lysodeikticus. J Biol Chem. 1956 Jul;221(1):413–423. [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Divalent cation activation of deoxycholate-solubilized and -inactivated membrane reduced nicotinamide adenine dinucleotide oxidase of Bacillus megaterium KM. J Bacteriol. 1970 Apr;102(1):172–177. doi: 10.1128/jb.102.1.172-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Masking of Bacillus megaterium KM membrane reduced nicotinamide adenine dinucleotide oxidase and solubilization studies. J Bacteriol. 1970 Apr;102(1):161–171. doi: 10.1128/jb.102.1.161-171.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Ishikawa S., Shimazono N. Respiratory chain and phosphorylation site of the sonicated membrane fragments of Micrococcus lysodeikticus. J Biochem. 1966 Feb;59(2):104–114. doi: 10.1093/oxfordjournals.jbchem.a128269. [DOI] [PubMed] [Google Scholar]
- Gel'man N. S., Tikhonova G. V., Simakova I. M., Lukoyanova M. A., Taptykova S. D., Mikelsaar H. M. Fragmentation by detergents of the respiratory chain of Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):321–331. doi: 10.1016/0005-2728(70)90188-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nachbar M. S., Salton M. R. Characteristics of a lipid-rich NADH dehydrogenase-containing particulate fraction obtained from Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):309–320. doi: 10.1016/0005-2728(70)90187-8. [DOI] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes; difference spectra. Arch Biochem Biophys. 1954 Jun;50(2):299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Factors affecting deoxycholate inactivation and Mg++ reactivation of Bacillus megaterium KM membrane nicotinamide adenine dinucleotide (reduced form) oxidase. J Bacteriol. 1970 Aug;103(2):467–474. doi: 10.1128/jb.103.2.467-474.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]