Abstract
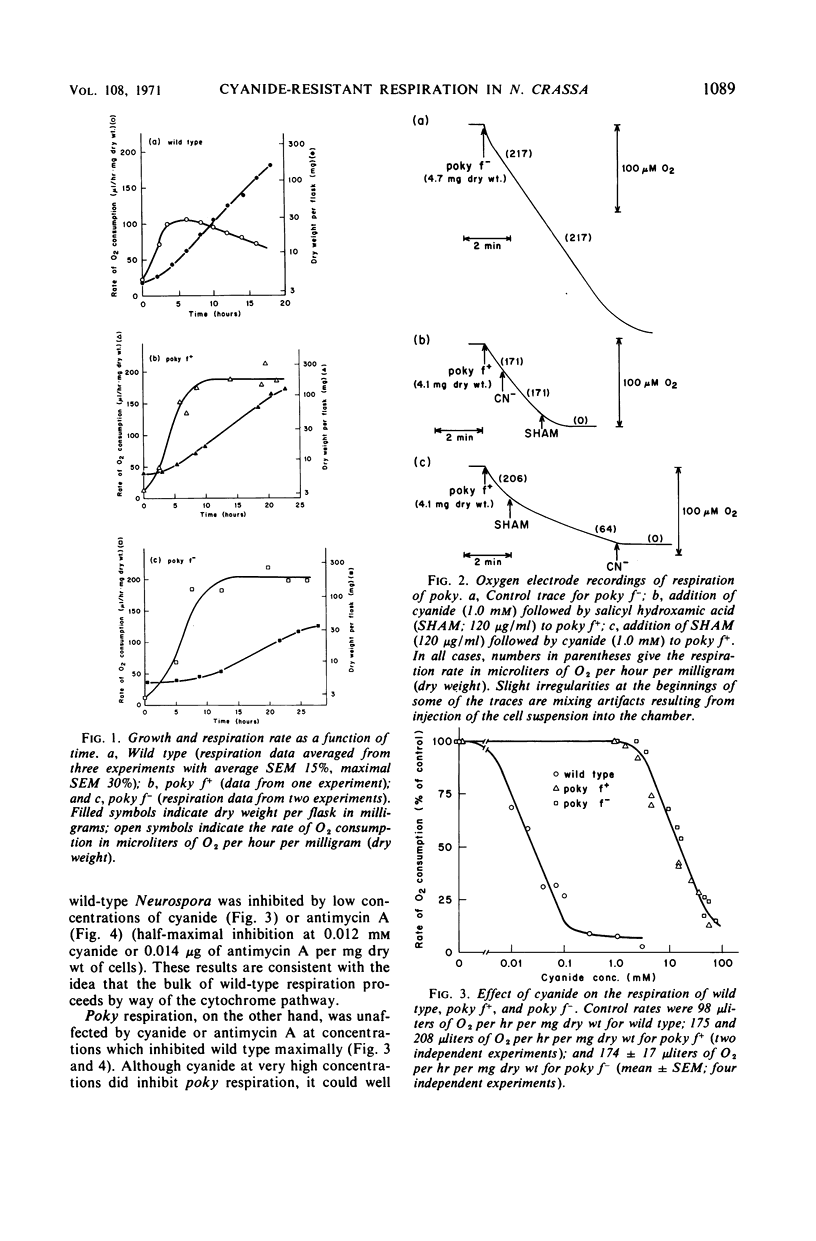
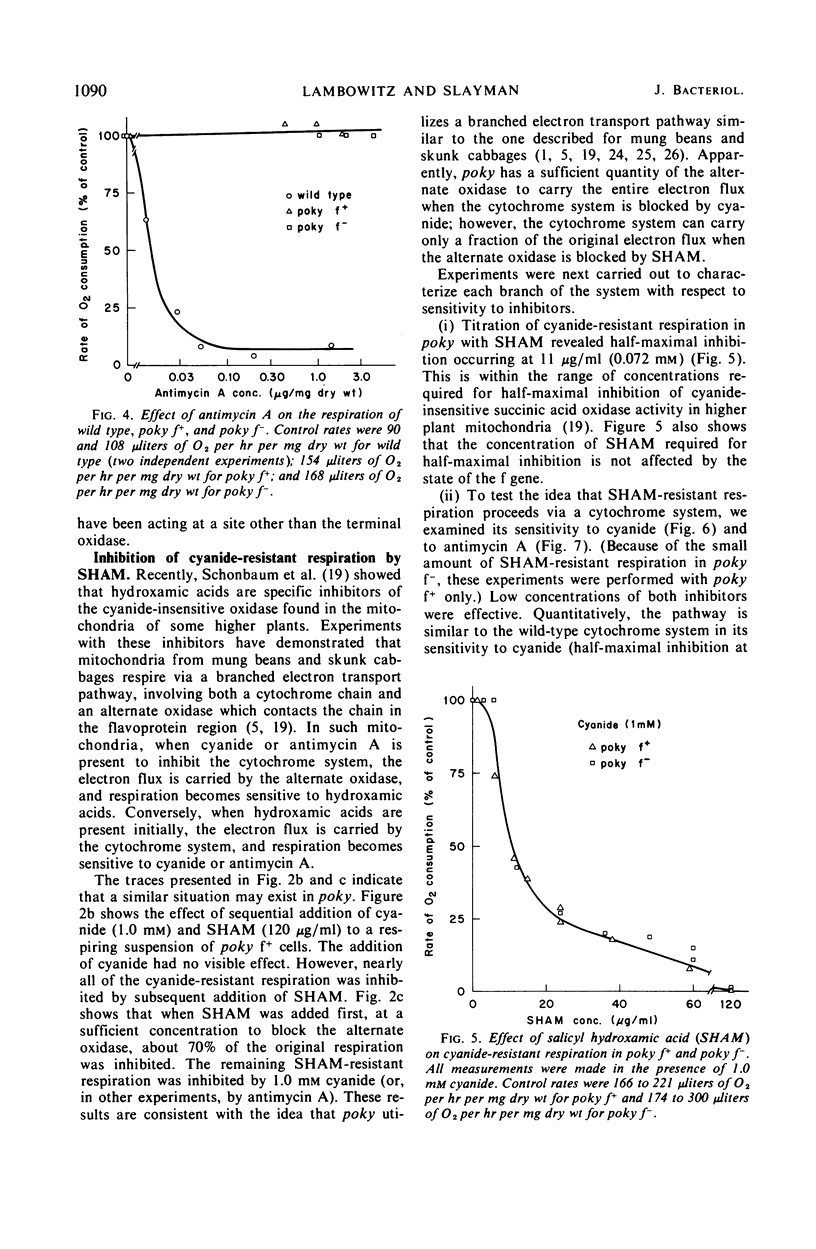
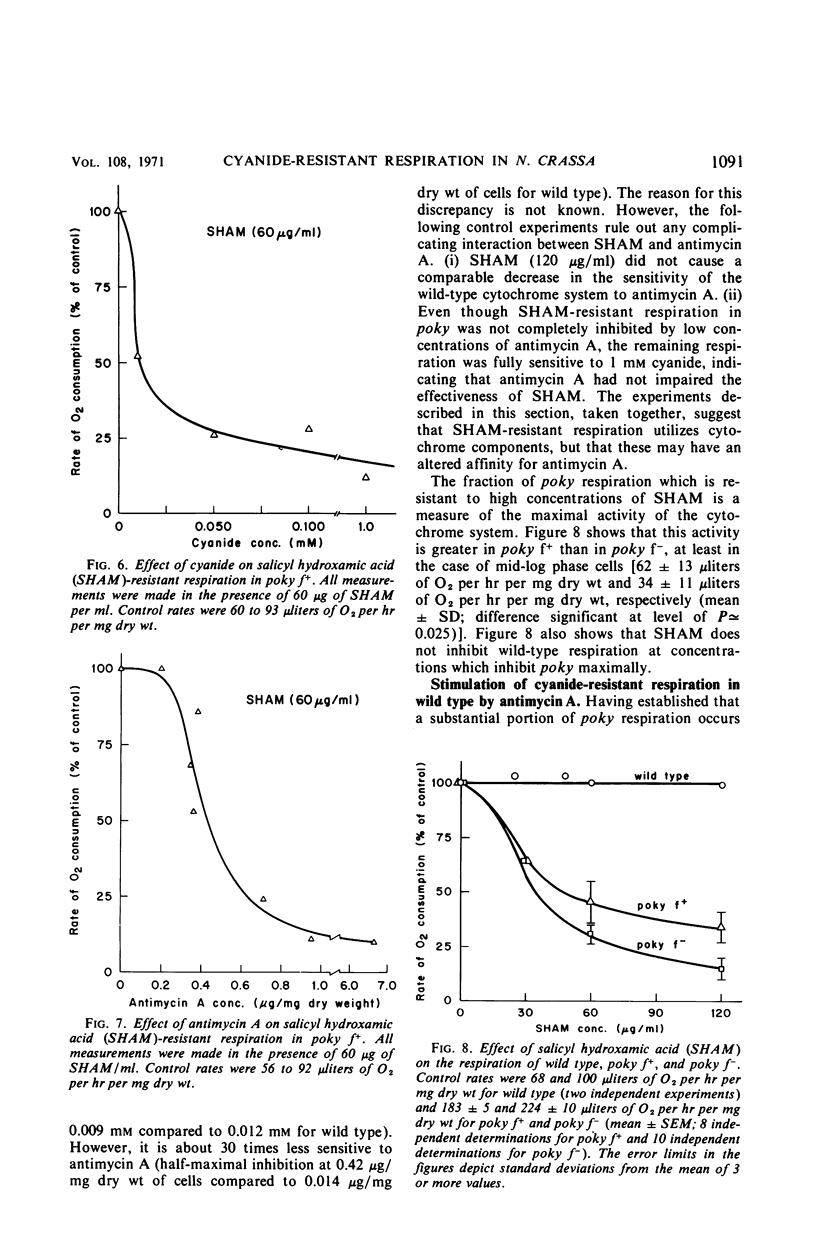
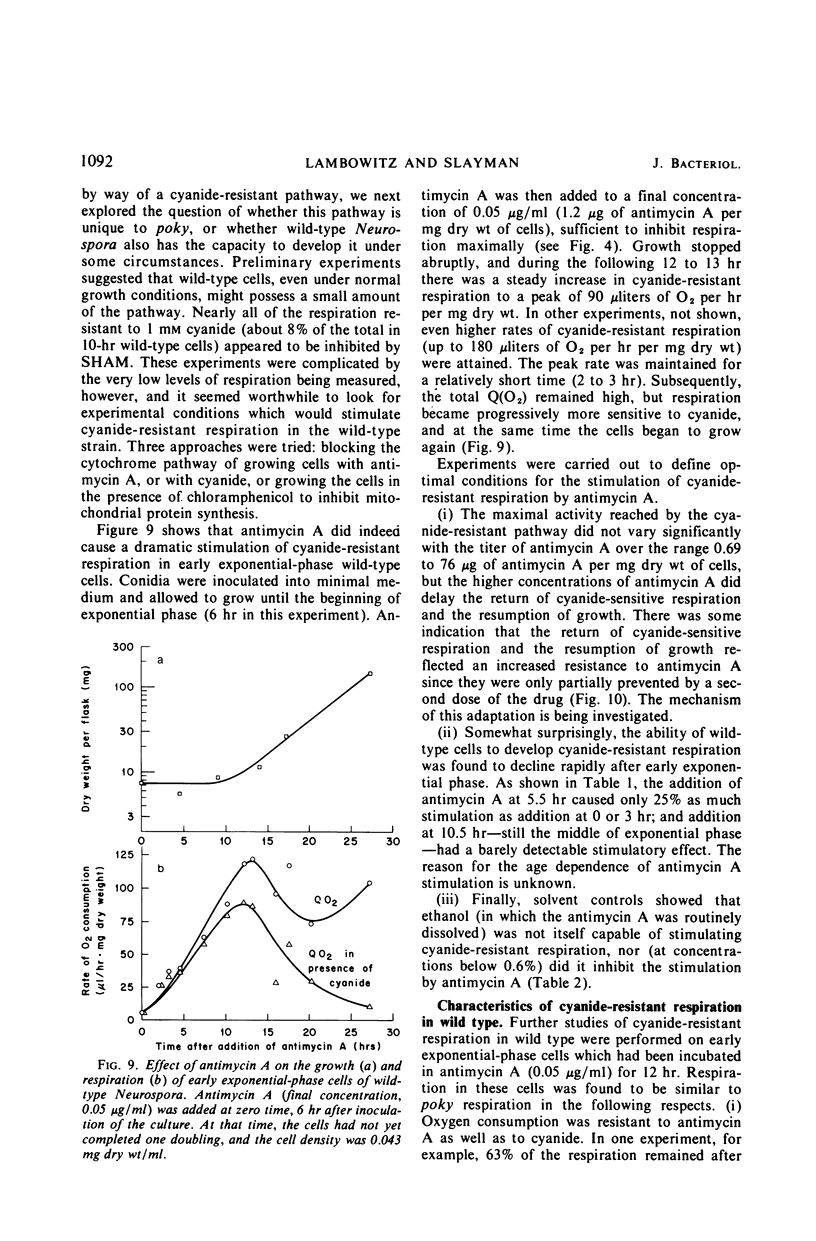
Cell respiration in wild type and poky was studied as part of a long-term investigation of cyanide-resistant respiration in Neurospora. Respiration in wild type proceeds via a cytochrome chain which is similar to that of higher organisms; it is sensitive to antimycin A or cyanide. Poky, on the other hand, respires by means of two alternative oxidase systems. One of these is analogous to the wild-type cytochrome chain in that it can be inhibited by antimycin A or cyanide; this system accounts for as much as 15% of the respiration of poky f− and 34% of the respiration of poky f+. The second oxidase system is unaffected by antimycin A or cyanide at concentrations which inhibit the cytochrome chain maximally. It can, however, be specifically inhibited by salicyl hydroxamic acid. The cyanide-resistant oxidase is not exclusive to poky, but is also present in small quantities in wild type grown under ordinary circumstances. These quantities may be greatly increased (as much as 20-fold) by growing wild type in the presence of antimycin A, cyanide, or chloramphenicol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Cytoplasmic Mutants Selected from Continuously Growing Cultures of NEUROSPORA CRASSA. Genetics. 1969 Mar;61(3):643–659. doi: 10.1093/genetics/61.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacumakos E. G., Garnjobst L., Tatum E. L. A cytoplasmic character in Neurospora crassa. The role of nuclei and mitochondria. J Cell Biol. 1965 Aug;26(2):427–443. doi: 10.1083/jcb.26.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin R. T., Mitchell H. K. Alterations of the respiratory system of Neurospora crassa by the mi-1 mutation. J Bacteriol. 1970 Oct;104(1):74–78. doi: 10.1128/jb.104.1.74-78.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnjobst L., Wilson J. F., Tatum E. L. Studies on a cytoplasmic character in Neurospora crassa. J Cell Biol. 1965 Aug;26(2):413–425. doi: 10.1083/jcb.26.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASKINS F. A., TISSIERES A., MITCHELL H. K., MITCHELL M. B. Cytochromes and the succinic acid oxidase system of poky strains of Neurospora. J Biol Chem. 1953 Feb;200(2):819–826. [PubMed] [Google Scholar]
- Hall D. O., Greenawalt J. W. The preparation and biochemical properties of mitochondria from Neurospora crassa. J Gen Microbiol. 1967 Sep;48(3):419–430. doi: 10.1099/00221287-48-3-419. [DOI] [PubMed] [Google Scholar]
- Kawakita M. Studies on the respiratory system of Aspergillus oryzae. 3. Properties of mitochondria from mycelia grown in the presence of antimycin A. J Biochem. 1971 Jan;69(1):35–42. doi: 10.1093/oxfordjournals.jbchem.a129457. [DOI] [PubMed] [Google Scholar]
- LUCK D. J. THE INFLUENCE OF PRECURSOR POOL SIZE ON MITOCHONDRIAL COMPOSITION IN NEUROSPORA CRASSA. J Cell Biol. 1965 Mar;24:445–460. doi: 10.1083/jcb.24.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- MITCHELL M. B., MITCHELL H. K. A nuclear gene suppressor of a cytoplasmically inherited character in Neurospora crassa. J Gen Microbiol. 1956 Feb;14(1):84–89. doi: 10.1099/00221287-14-1-84. [DOI] [PubMed] [Google Scholar]
- McDougall K. J., Pittenger T. H. A cytoplasmic variant of Neurospora crassa. Genetics. 1966 Aug;54(2):551–565. doi: 10.1093/genetics/54.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K. A Case of "Maternal" Inheritance in Neurospora Crassa. Proc Natl Acad Sci U S A. 1952 May;38(5):442–449. doi: 10.1073/pnas.38.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K., Tissieres A. Mendelian and Non-Mendelian Factors Affecting the Cytochrome System in Neurospora Crassa. Proc Natl Acad Sci U S A. 1953 Jul;39(7):606–613. doi: 10.1073/pnas.39.7.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin M. R., Luck D. J. Defective production of mitochondrial ribosomes in the poky mutant of Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Feb;68(2):287–290. doi: 10.1073/pnas.68.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- SRB A. M. Some consequences of nuclear-cytoplasmic recombinations among various neurosporas. Cold Spring Harb Symp Quant Biol. 1958;23:269–277. doi: 10.1101/sqb.1958.023.01.028. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., MITCHELL H. K. Cytochromes and respiratory activities in some slow growing strains of Neurospora. J Biol Chem. 1954 May;208(1):241–249. [PubMed] [Google Scholar]
- TISSIERES A., MITCHELL H. K., HASKINS F. A. Studies on the respiratory system of the poky strain of Neurospora. J Biol Chem. 1953 Nov;205(1):423–433. [PubMed] [Google Scholar]
- Weiss H., von Jagow G., Klingenberg M., Bücher T. Characterization of Neurospora crassa mitochondria prepared with a grind-mill. Eur J Biochem. 1970 May 1;14(1):75–82. doi: 10.1111/j.1432-1033.1970.tb00263.x. [DOI] [PubMed] [Google Scholar]


