Abstract
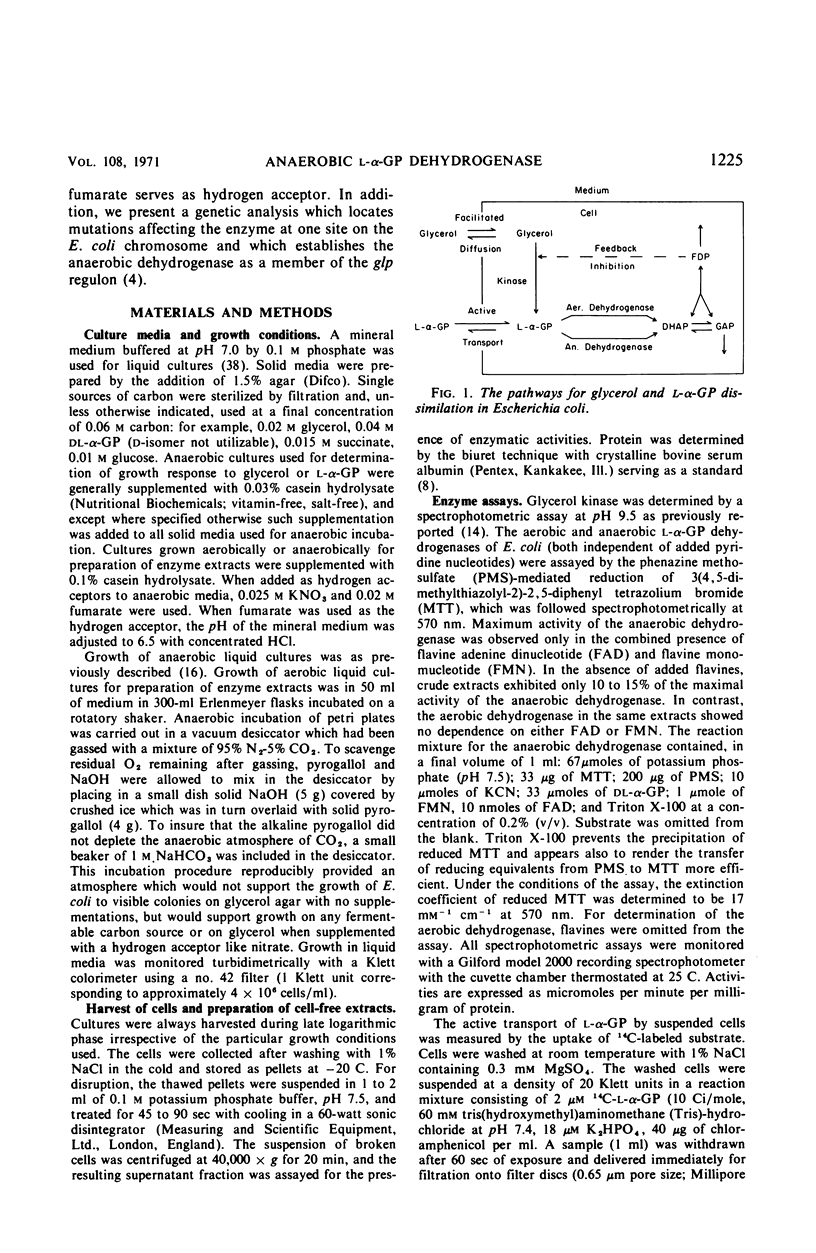
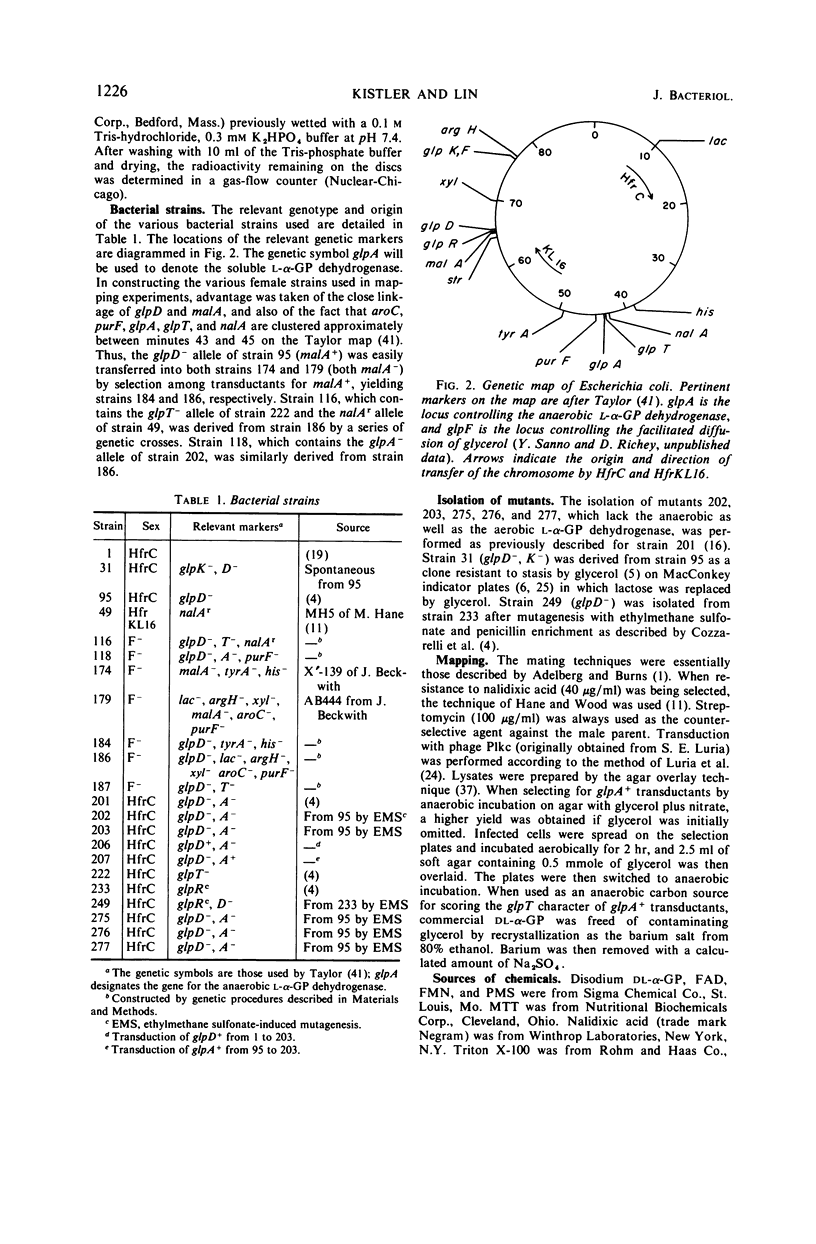
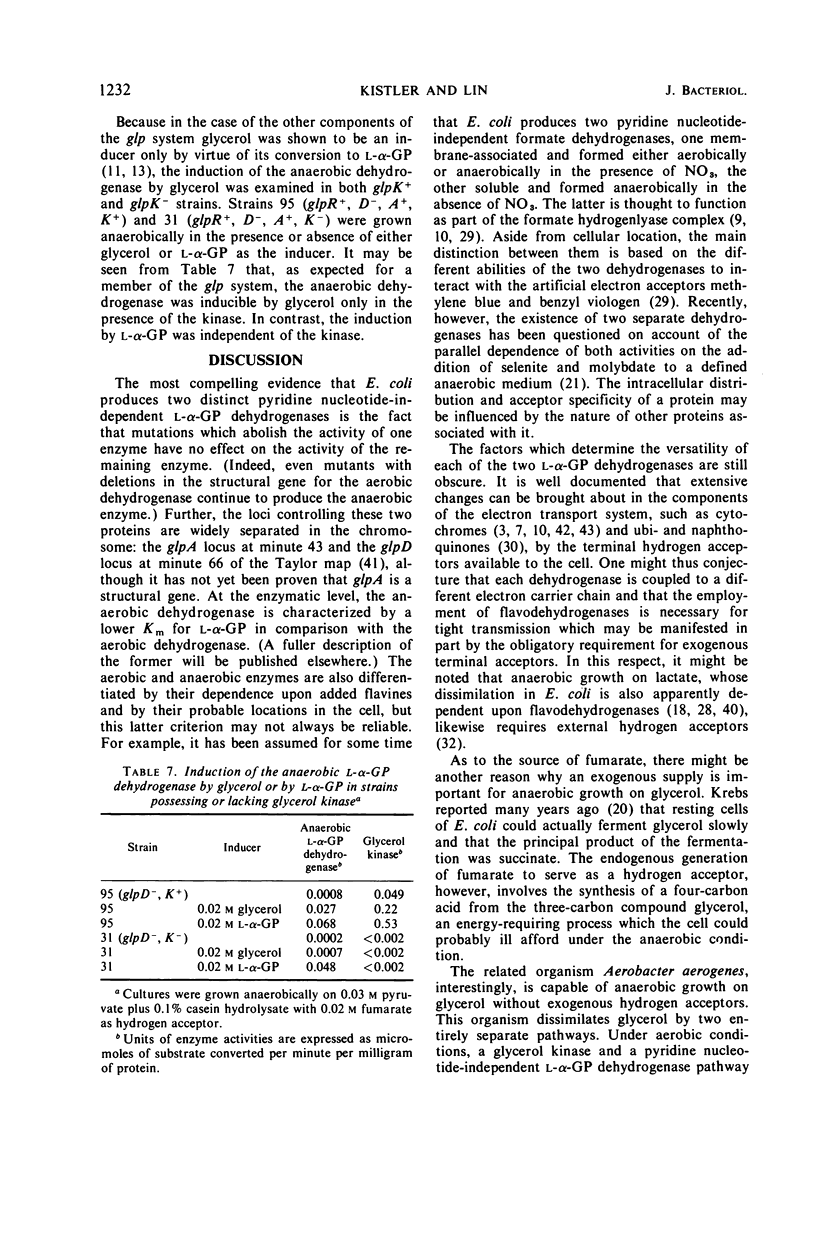
In mutant cells of Escherichia coli missing the particulate l-α-glycerophosphate (l-α-GP) dehydrogenase necessary for aerobic growth on glycerol or l-αGP, a soluble, flavine-dependent l-α-GP dehydrogenase supports normal anaerobic growth rates on either of the two substrates with fumarate or nitrate as exogenous hydrogen acceptor. In an experiment in which glycerol served as the carbon source and nitrate as the acceptor, the growth of such a mutant was arrested upon the admission of air, whereas the growth of wild-type cells continued smoothly. Mutant cells lacking the soluble l-α-GP dehydrogenase, but possessing the particulate enzyme, can grow at normal rates aerobically on glycerol and l-α-GP or anaerobically on these compounds with nitrate, but not fumarate, as the hydrogen acceptor. Double mutants lacking both of the dehydrogenases fail to show significant growth on either glycerol or l-α-GP under any condition. Mutations affecting the anaerobic dehydrogenase (glpA locus) are situated at about minute 43 of the Taylor map, just clockwise beyond glpT, and show cotransduction with purF (1.5%), glpT (91%), and nalA (50%). The anaerobic dehydrogenase is a member of the glp regulon as judged by its inducibility by l-α-GP and by its constitutive formation in strains of glpRc genotype. The level of the anaerobic dehydrogenase is about the same in cells grown either aerobically or anaerobically with nitrate serving as a terminal hydrogen acceptor. With fumarate as terminal acceptor, the level is elevated several fold.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Purification and properties of glycerol kinase from Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):1030–1035. [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Purification and regulatory properties of the biosynthetic L-glycerol 3-phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3316–3323. [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The role of fumarate in the respiration of Bacterium coli commune. Biochem J. 1937 Nov;31(11):2095–2124. doi: 10.1042/bj0312095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASON A. Symposium on metabolism of inorganic compounds. II. Enzymatic pathways of nitrate, nitrite, and hydroxylamine metabolisms. Bacteriol Rev. 1962 Mar;26:16–41. doi: 10.1128/br.26.1.16-41.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTA A., YAMANAKA T., OKUNUKI K. OXIDATIVE PHOSPHORYLATION COUPLED WITH NITRATE RESPIRATION. II. PHOSPHORYLATION COUPLED WITH ANAEROBIC NITRATE REDUCTION IN A CELL-FREE EXTRACT OF ESCHERICHIA COLI. J Biochem. 1964 Feb;55:131–135. [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal M. C., Puig J., Lepelletier M. Etude génétique d'une mutation affectant l'activité L-actate-déshydrogénase chez escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 27;268(4):737–739. [PubMed] [Google Scholar]
- Polglase W. J., Pun W. T., Withaar J. Lipoquinones of Escherichia coli. Biochim Biophys Acta. 1966 May 5;118(2):425–426. doi: 10.1016/s0926-6593(66)80053-x. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M. Further Observations on the Anaerobic Growth of Bacteria. Biochem J. 1925;19(4):660–666. doi: 10.1042/bj0190660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M., Whetham M. D. Some Reactions of Resting Bacteria in Relation to Anaerobic Growth. Biochem J. 1925;19(2):304–317. doi: 10.1042/bj0190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSH D., KARIBIAN D., KARNOVSKY M. L., MAGASANIK B. Pathways of glycerol dissimilation in two strains of Aerobacter aerogenes; enzymatic and tracer studies. J Biol Chem. 1957 Jun;226(2):891–899. [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]


