Abstract
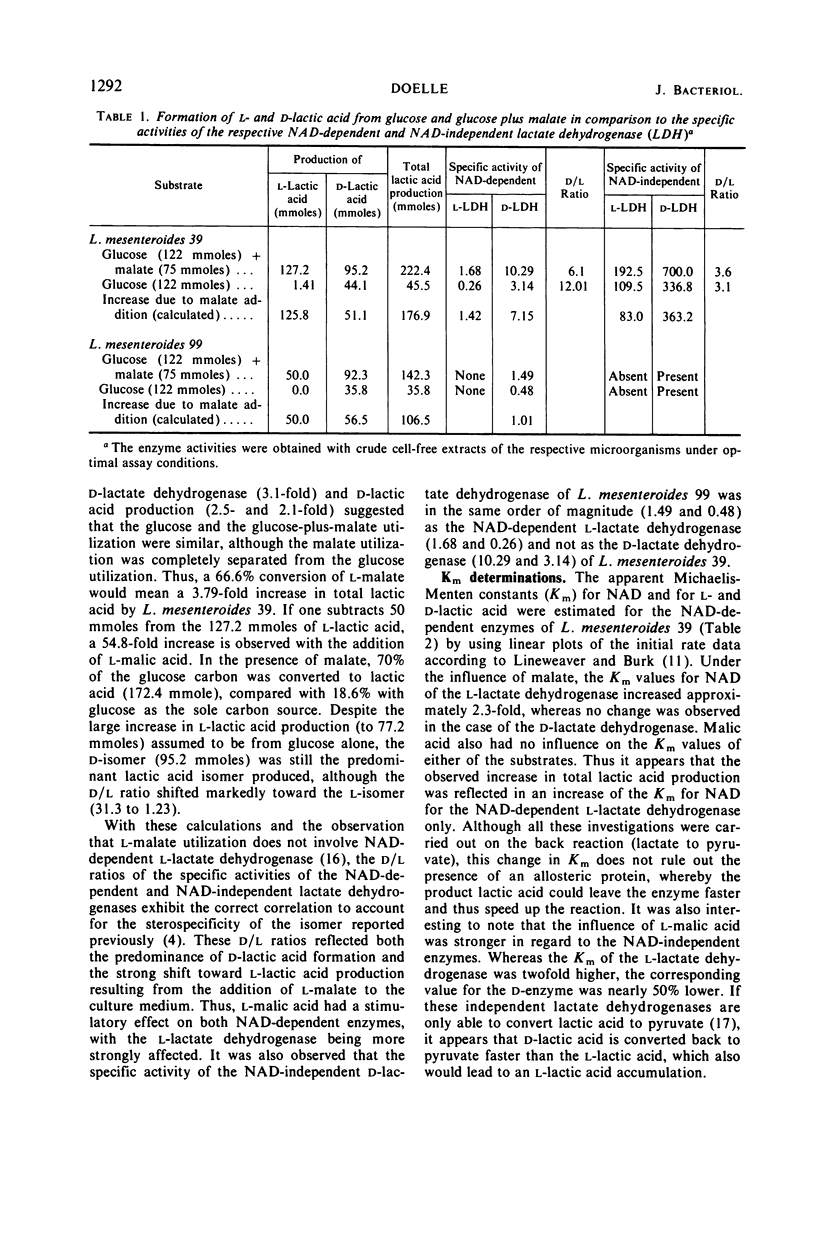
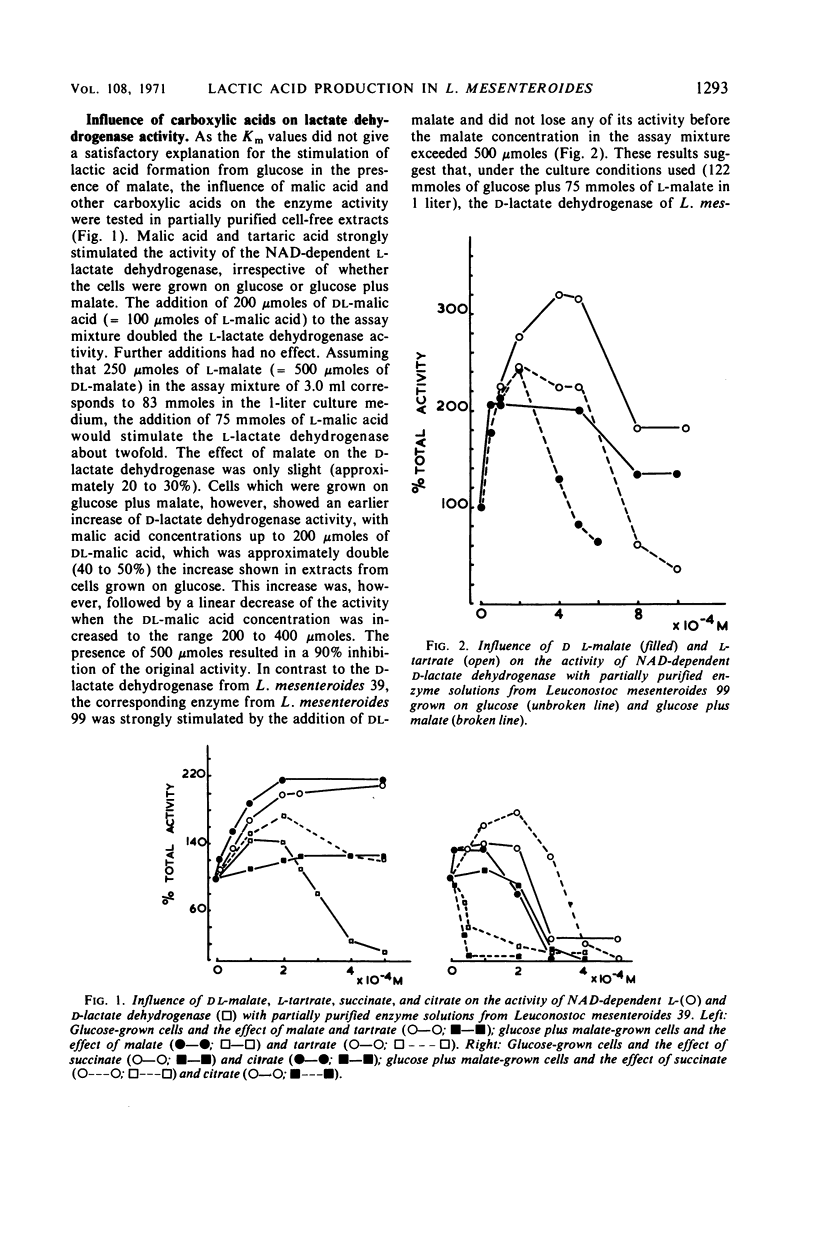
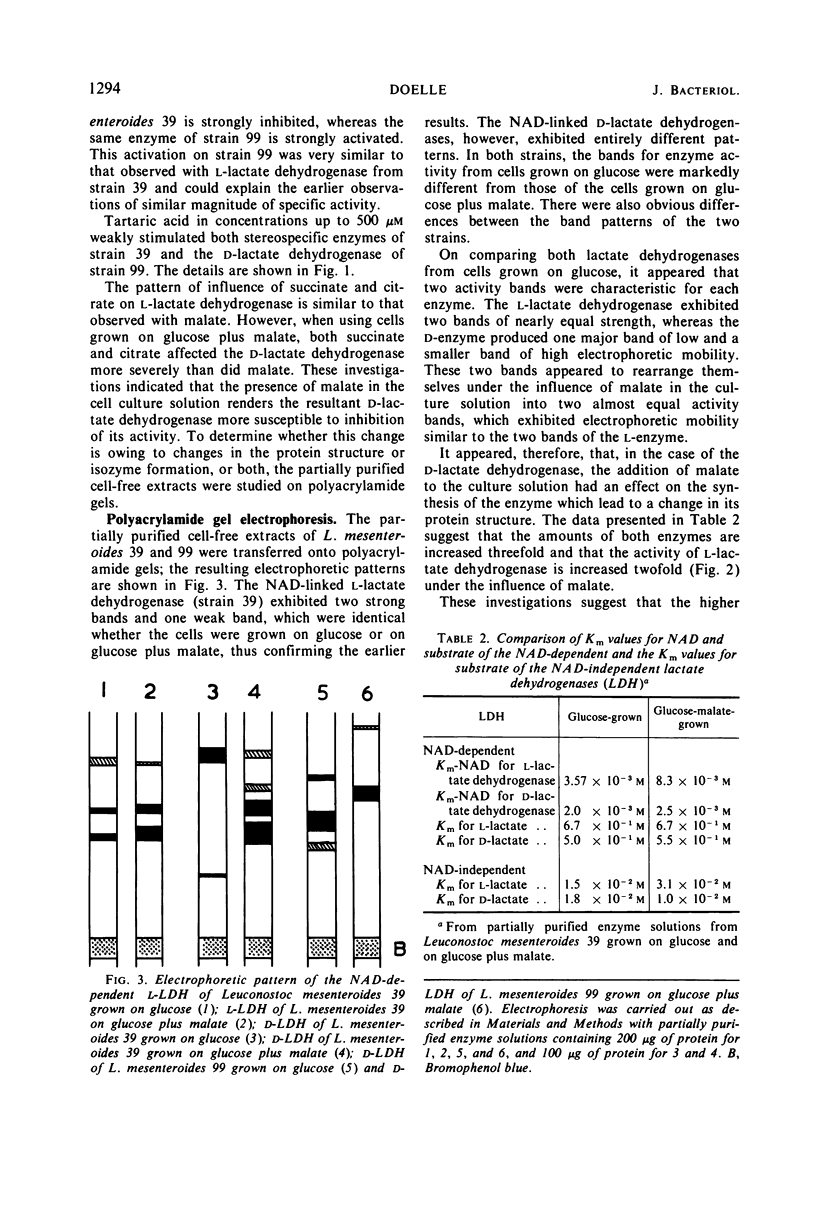
Leuconostoc mesenteroides increased its lactic acid production from glucose threefold when malic acid was added to the culture. This increase resulted also in a reduction of the ratio of d-lactic acid to l-lactic acid (31.5 to 1.23). Addition of malic acid increased 6.5-fold the specific activity of nicotinamide adenine dinucleotide (NAD)-linked l-lactate dehydrogenase and increased 3.2-fold that of NAD-linked d-lactate dehydrogenase. The Michaelis constant (Km) for NAD of the NAD-linked l-lactate dehydrogenase increased with the addition of malate, but no change was observed in the Km values for the respective d-enzyme. The effect of carboxylic acids on the NAD-linked l-lactate dehydrogenase activities was tested by using partially purified enzyme preparations from cells grown with glucose alone and from cells grown with glucose plus malate. Malate stimulated the l-enzyme and inhibited the d-lactate dehydrogenase. The NAD-linked l-lactate dehydrogenase exhibited the same activity bands on polyacrylamide gel electrophoresis whether the cell-free preparation originated from cells grown on glucose plus malate or on glucose as the sole carbon source. The NAD-linked d-lactate dehydrogenase, however, exhibited a different pattern of electrophoretic mobility, depending upon the source of origin of the cell-free preparation. The results suggest that malate has a stimulatory effect on the synthesis of both enzymes and may result in rearrangement of the protein structure of the d-lactate dehydrogenase. This rearrangement apparently makes the d-enzyme more susceptible to inhibition of catalytic activity. The l-lactate dehydrogenase, however, is stimulated not only in its synthesis but also in its activity. It is proposed that these effects are responsible for the regulation of lactic acid production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amerine M. A., Kunker R. E. Micobiology of winemaking. Annu Rev Microbiol. 1968;22:323–358. doi: 10.1146/annurev.mi.22.100168.001543. [DOI] [PubMed] [Google Scholar]
- Doelle H. W. Nicotinamide adenine dinucleotide-dependent and nicotinamide adenine dinucleotide-independent lactate dehydrogenases in homofermentative and heterofermentative lactic acid bacteria. J Bacteriol. 1971 Dec;108(3):1284–1289. doi: 10.1128/jb.108.3.1284-1289.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch P. Morphologie, Stoffwechselphysiologie und Charakterisierung der Malic-Enzym-Aktivität L-Apfelsäure-abbauender Bakterien. Arch Mikrobiol. 1968;60(4):285–302. [PubMed] [Google Scholar]
- Flesch P. Uber die Malat-Dehydrogenase und Lactat-Dehydrogenase-Aktivität L-Apfelsäure-abbauender Bakterien. Arch Mikrobiol. 1969;68(3):259–277. [PubMed] [Google Scholar]
- Garvie E. I. Lactic dehydrogenases of strains of the genus Leuconostoc. J Gen Microbiol. 1969 Sep;58(1):85–94. doi: 10.1099/00221287-58-1-85. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S., KORKES S., DEL CAMPILLO A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. V. Further study of the "malic" enzyme of Lactobacillus arabinosus. J Biol Chem. 1951 Sep;192(1):301–312. [PubMed] [Google Scholar]
- Kunkee R. E. Malo-lactic fermentation. Adv Appl Microbiol. 1967;9:235–279. doi: 10.1016/s0065-2164(08)70530-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SNOSWELL A. M. OXIDIZED NICOTINAMIDE-ADENINE DINUCLEOTIDE-INDEPENDENT LACTATE DEHYDROGENASES OF LACTOBACILLUS ARABINOSUS 17.5. Biochim Biophys Acta. 1963 Sep 3;77:7–9. doi: 10.1016/0006-3002(63)90464-5. [DOI] [PubMed] [Google Scholar]


