Abstract
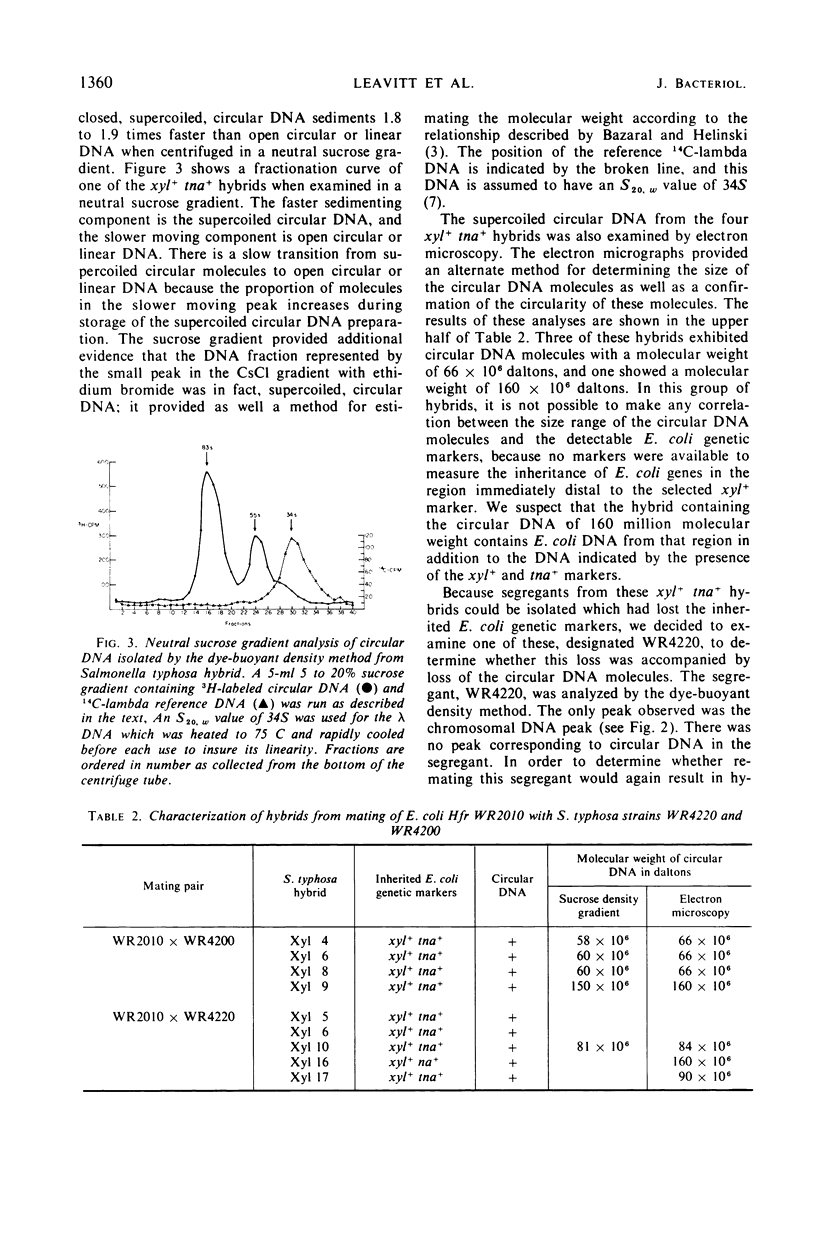
Heterozygous, partial diploid Salmonella typhosa hybrids obtained from matings with Escherichia coli K-12 Hfr strains were observed to contain supercoiled, circular deoxyribonucleic acid (DNA) when examined by the dye-buoyant density method. Examination of one such S. typhosa hybrid after its loss, by segregation, of the inherited E. coli genetic markers revealed a concurrent loss of its supercoiled circular DNA. Subsequent remating of this segregant with various E. coli Hfr strains resulted in the reappearance of the circular DNA. Molecular weight determinations of circular DNA molecules isolated from a number of S. typhosa partial diploid hybrids were made by sucrose density gradient ultracentrifugation and electron microscopy. These studies revealed a range of molecular sizes among the various hybrids examined, but each hybrid exhibited only a single characteristic size for its contained circular DNA. The range of size is consistent with the presence in each hybrid of a different length of E. coli chromosome. It was concluded that the E. coli Hfr genetic segments transferred to these S. typhosa hybrids were conserved, in the diploid state, in the form of supercoiled, circular DNA molecules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON L. S., SPILMAN W. M., CAREY W. F. Diploid heterozygous hybrids from matings between Escherichia coli and Salmonella typhosa. J Exp Med. 1960 Aug 1;112:361–372. doi: 10.1084/jem.112.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Gemski P., Johnson E. M., Wohlhieter J. A. Intergeneric bacterial matings. Bacteriol Rev. 1968 Dec;32(4 Pt 1):362–369. [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Easterling S. B., Johnson E. M., Wohlhieter J. A., Baron L. S. Nature of lactose-fermenting Salmonella strains obtained from clinical sources. J Bacteriol. 1969 Oct;100(1):35–41. doi: 10.1128/jb.100.1.35-41.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Rownd R., Baron L. S. GENETIC HOMOLOGY BETWEEN ESCHERICHIA COLI K-12 AND SALMONELLA. J Bacteriol. 1962 Dec;84(6):1303–1312. doi: 10.1128/jb.84.6.1303-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. IV. Molecular weights of the DNA of several F' elements. J Mol Biol. 1968 Jul 14;35(1):95–102. doi: 10.1016/s0022-2836(68)80039-7. [DOI] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. M., FALKOW S., BARON L. S. RECIPIENT ABILITY OF SALMONELLA TYPHOSA IN GENETIC CROSSES WITH ESCHERICHIA COLI. J Bacteriol. 1964 Jan;87:54–60. doi: 10.1128/jb.87.1.54-60.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Easterling S. B., Baron L. S. Conservation and transfer of Escherichia coli genetic segments by partial diploid Hfr strains of Salmonella typhosa. J Bacteriol. 1970 Nov;104(2):668–673. doi: 10.1128/jb.104.2.668-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Easterling S. B., Baron L. S. Inefficiency of genetic recombination in hybrids between Escherichia coli and Salmonella typhosa. J Bacteriol. 1971 Apr;106(1):243–249. doi: 10.1128/jb.106.1.243-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. F., Helinski D. R. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):650–657. doi: 10.1073/pnas.58.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]





