Abstract
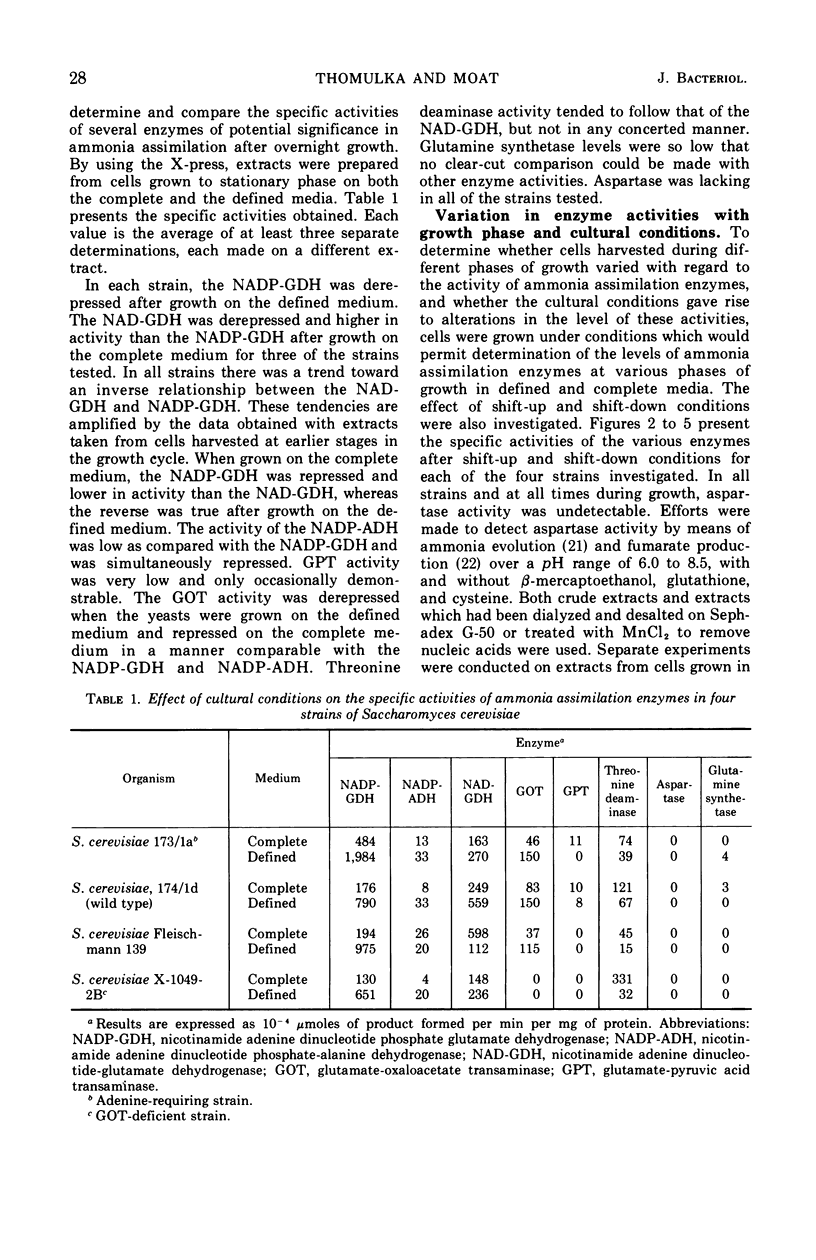
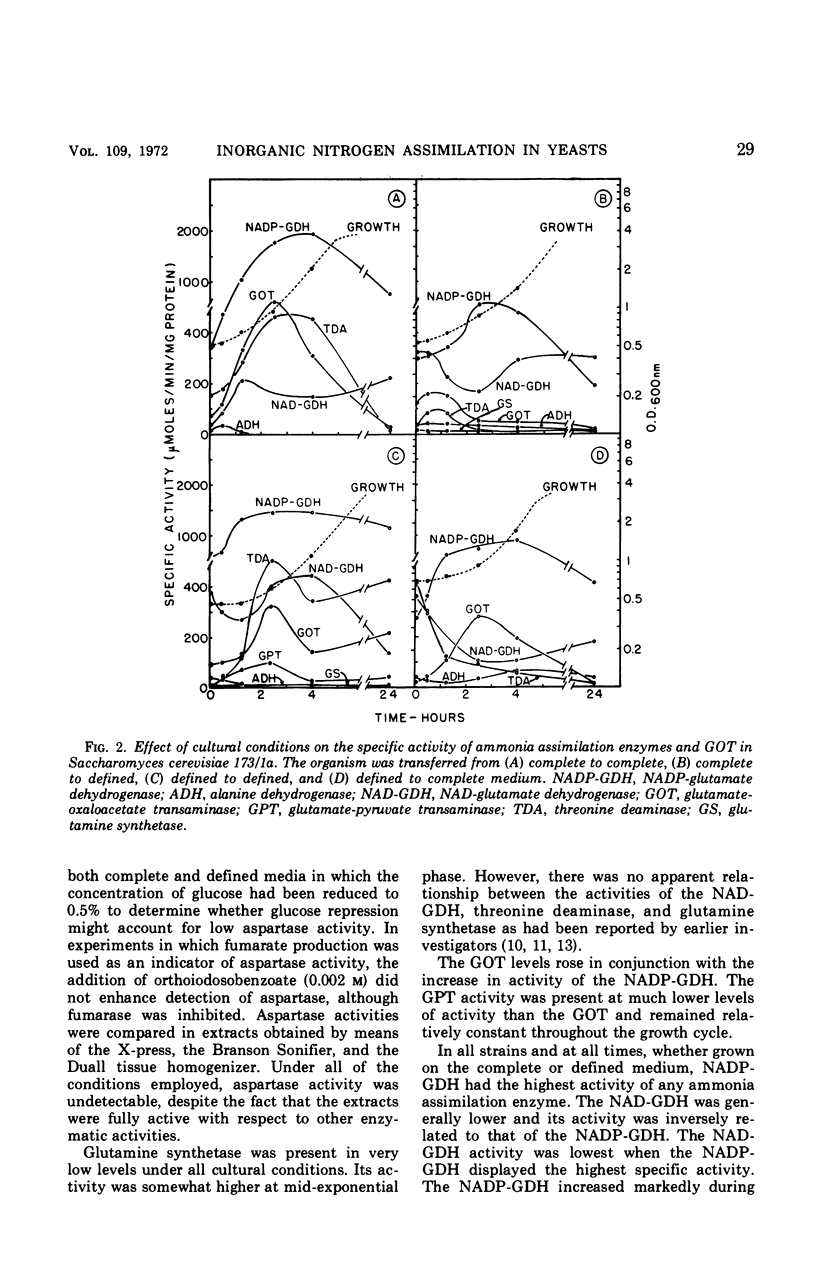
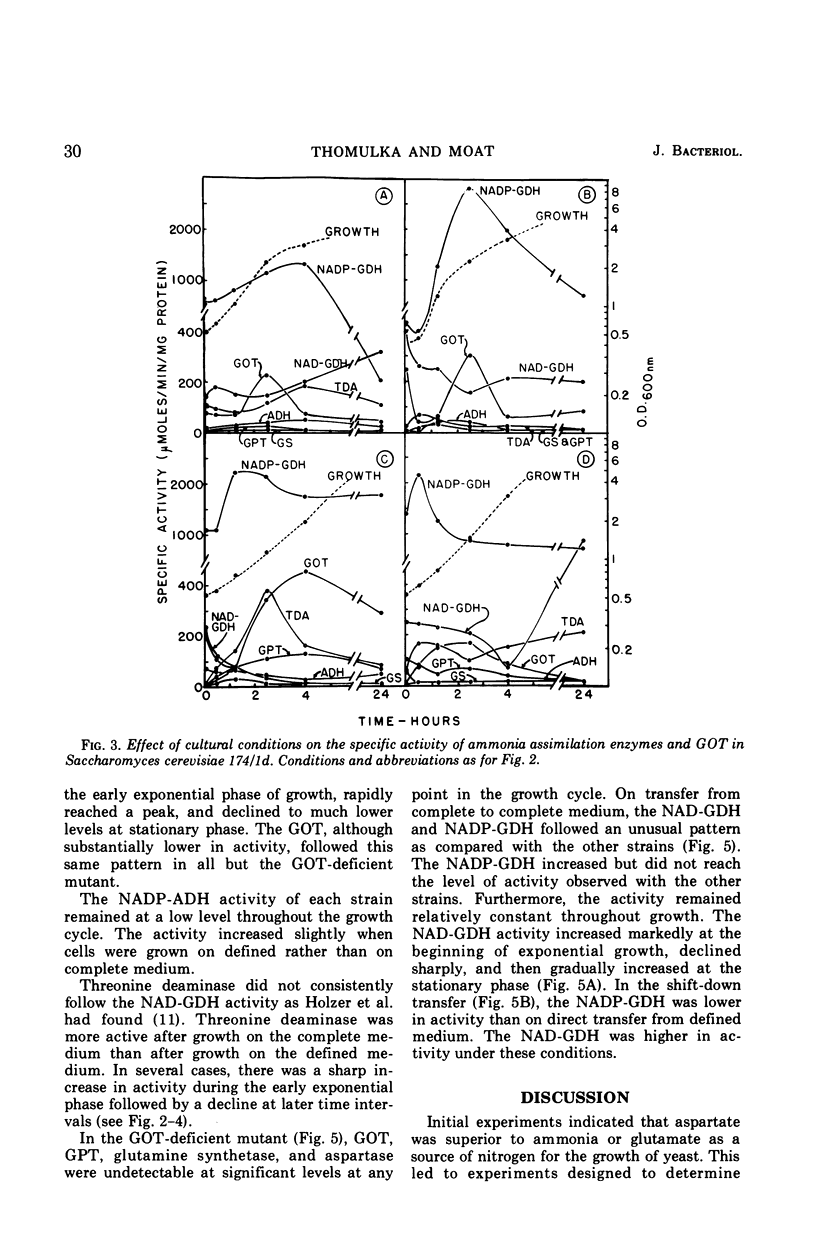
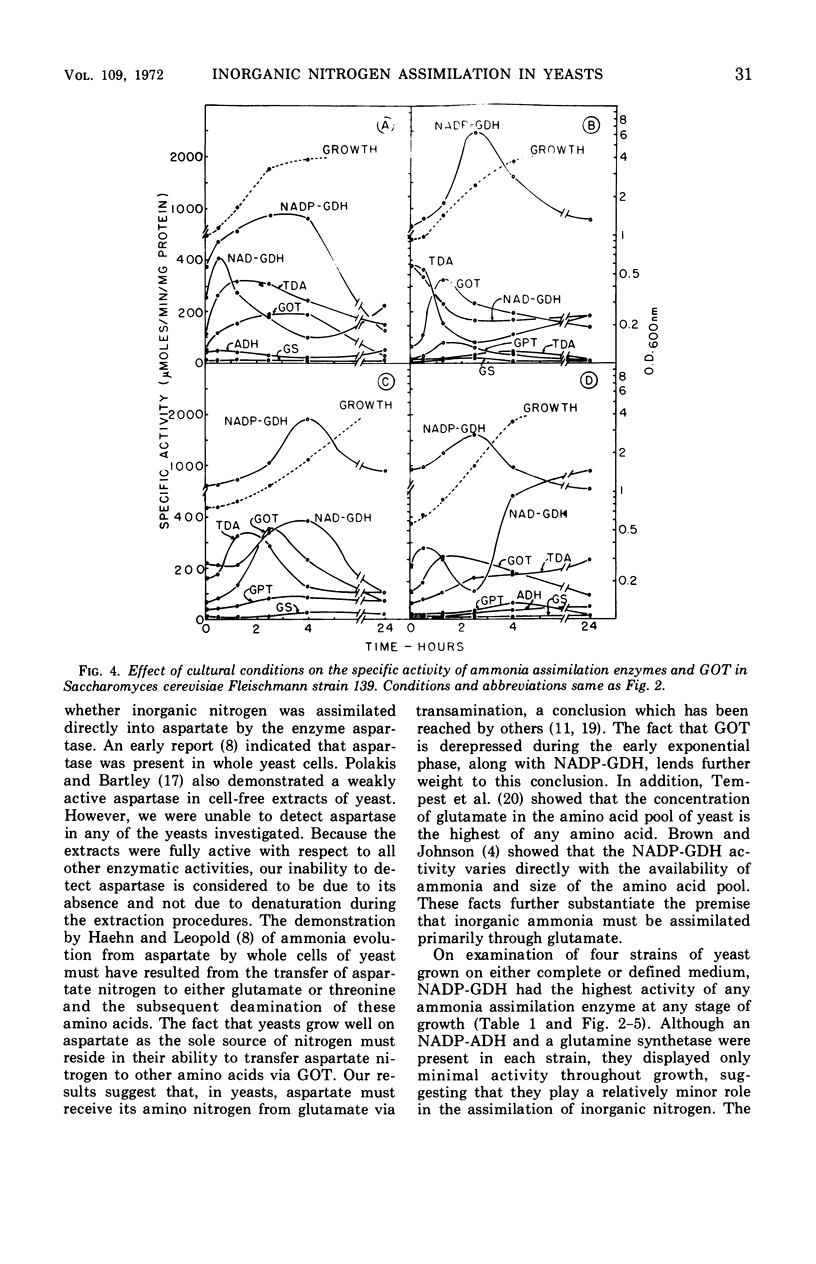
Ammonia assimilation has been investigated in four strains of Saccharomyces cerevisiae by measuring, at intervals throughout the growth cycle, the activities of several enzymes concerned with inorganic ammonia assimilation. Enzyme activities in extracts of cells were compared after growth in complete and defined media. The effect of shift from growth in a complete to growth in a defined medium (and the reverse) was also determined. The absence of aspartase (EC 4.3.1.1, l-aspartate-ammonia lyase) activity, the low specific activities of alanine dehydrogenase, glutamine synthetase [EC 6.3.1.2, l-glutamate-ammonia ligase (ADP)], and the marked increase in activity of the nicotinamide adenine dinucleotide phosphate-linked glutamate dehydrogenase (NADP-GDH) [EC 1.4.1.4, l-glutamate:NADP-oxidoreductase (deaminating)] during the early stages of growth support the conclusion that yeasts assimilate ammonia primarily via glutamate. The NADP-GDH showed a rapid increase in activity just before the initiation of exponential growth, reached a maximum at the mid-exponential stage, and then gradually declined in activity in the stationary phase. The NADP-GDH reached a higher level of activity when the yeasts were grown on the defined medium as compared with complete medium. The nicotinamide adenine dinucleotide-linked glutamate dehydrogenase (NAD-GDH) [EC 1.4.1.2, l-glutamate:NAD-oxidoreductase (deaminating)] showed only slight increases in activity during the exponential phase of growth. There was an inverse relationship in that the NADP-GDH increased in activity as the NAD-GDH decreased. The NAD-GDH activity was higher after growth on the complete medium. The glutamate-oxaloacetate transaminase (EC 2.6.1.1. l-aspartate:2-oxoglutarate aminotransferase) activity rose and fell in parallel with the NADP-GDH, although its specific activity was somewhat lower. Although other ammonia-assimilatory enzymes were demonstrable, it seems unlikely that their combined activities could account for the remainder of the ammonia-assimilatory capacity not accounted for by the NADP-GDH. The ability of aspartate to serve as effectively as glutamate as the sole source of nitrogen for the growth of yeast apparently resides in their ability to utilize aspartate for amino acid biosynthesis via transamination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Moat A. G. Nicotinic acid biosynthesis in prototrophs and tryptophan auxotrophs of Saccharomyces cerevisiae. J Biol Chem. 1966 Feb 25;241(4):775–780. [PubMed] [Google Scholar]
- BARRATT R. W., STRICKLAND W. N. Purification and characterization of a TPN-specific glutamic acid dehydrogenase Neurospora crassa. Arch Biochem Biophys. 1963 Jul;102:66–76. doi: 10.1016/0003-9861(63)90321-7. [DOI] [PubMed] [Google Scholar]
- BIGGER-GEHRING L. Transamination reactions in Saccharomyces fragilis. J Gen Microbiol. 1955 Aug;13(1):45–53. doi: 10.1099/00221287-13-1-45. [DOI] [PubMed] [Google Scholar]
- BURK R. R., PATEMAN J. A. Glutamic and alanine dehydrogenase determined by one gene in Neurospora crassa. Nature. 1962 Nov 3;196:450–451. doi: 10.1038/196450a0. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Johnson B. Influence of the concentration of glucose and galactose on the physiology of Saccharomyces cerevisiae in continuous culture. J Gen Microbiol. 1970 Dec;64(3):279–287. doi: 10.1099/00221287-64-3-279. [DOI] [PubMed] [Google Scholar]
- FINCHAM J. R., STADLER D. R. COMPLEMENTATION RELATIONSHIP OF NEUROSPORA AM MUTANTS IN RELATION TO THEIR FORMATION OF ABNORMAL VARIETIES OF GLUTAMATE DEHYDROGENASE. Genet Res. 1965 Feb;6:121–129. doi: 10.1017/s0016672300003980. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLHAW G., DRAEGERT W., HOLZER H. PARALLEL-REPRESSION DER SYNTHESE VON GLUTAMIN-SYNTHETASE UND DPN-ABHAENGIGER GLUTAMAT-DEHYDROGENASE IN HEFE. Biochem Z. 1965 Feb 8;341:224–238. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Bernlohr R. W. Purification, properties, and regulation of glutamic dehydrogenase of Bacillus licheniformis. J Bacteriol. 1971 May;106(2):375–385. doi: 10.1128/jb.106.2.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANWAL B. D. Diphosphopyridine nucleotide and triphosphopyridine nucleotide linked glutamic dehydrogenases of Fusarium. Arch Biochem Biophys. 1961 May;93:377–386. doi: 10.1016/0003-9861(61)90281-8. [DOI] [PubMed] [Google Scholar]
- SIMS A. P., FOLKES B. F. A KINETIC STUDY OF THE ASSIMILATION OF (15N)-AMMONIA AND THE SYNTHESIS OF AMINO ACIDS IN AN EXPONENTIALLY GROWING CULTURE OF CANDIDA UTILIS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:479–502. doi: 10.1098/rspb.1964.0015. [DOI] [PubMed] [Google Scholar]
- TERNBERG J. L., HERSHEY F. B. Colorimetric determination of blood ammonia. J Lab Clin Med. 1960 Nov;56:766–776. [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Williams V. R., Lartigue D. J. Quaternary structure and certain allosteric properties of aspartase. J Biol Chem. 1967 Jun 25;242(12):2973–2978. [PubMed] [Google Scholar]


