Abstract
Phosphoenolpyruvate (PEP) carboxylase was purified over 400-fold from Plasmodium berghei. The purified enzyme was stable in 0.4 m potassium phosphate buffer (pH 7.4) containing 0.5 m glucose, 1 mm ethylenediaminetetraacetic acid (EDTA), and 1 mm MgCl2. It had a molecular weight of 280,000 determined by sucrose density gradient centrifugation in this buffer, but it aggregated and was unstable in the presence of different salts or a more dilute solution of potassium phosphate. The Km for PEP was 2.6 mm and that for Mg2+ was 1.3 mm. The Km for bicarbonate was 2 mm. Citrate, nucleotides, and EDTA inhibited the PEP carboxylase of P. berghei by decreasing the concentration of free magnesium ions, but acetyl-coenzyme A, fructose-1,6-diphosphate, and aspartate did not influence its activity. A chloroquine concentration of 1.8 × 10−4m inhibited the enzyme 50%.
Full text
PDF

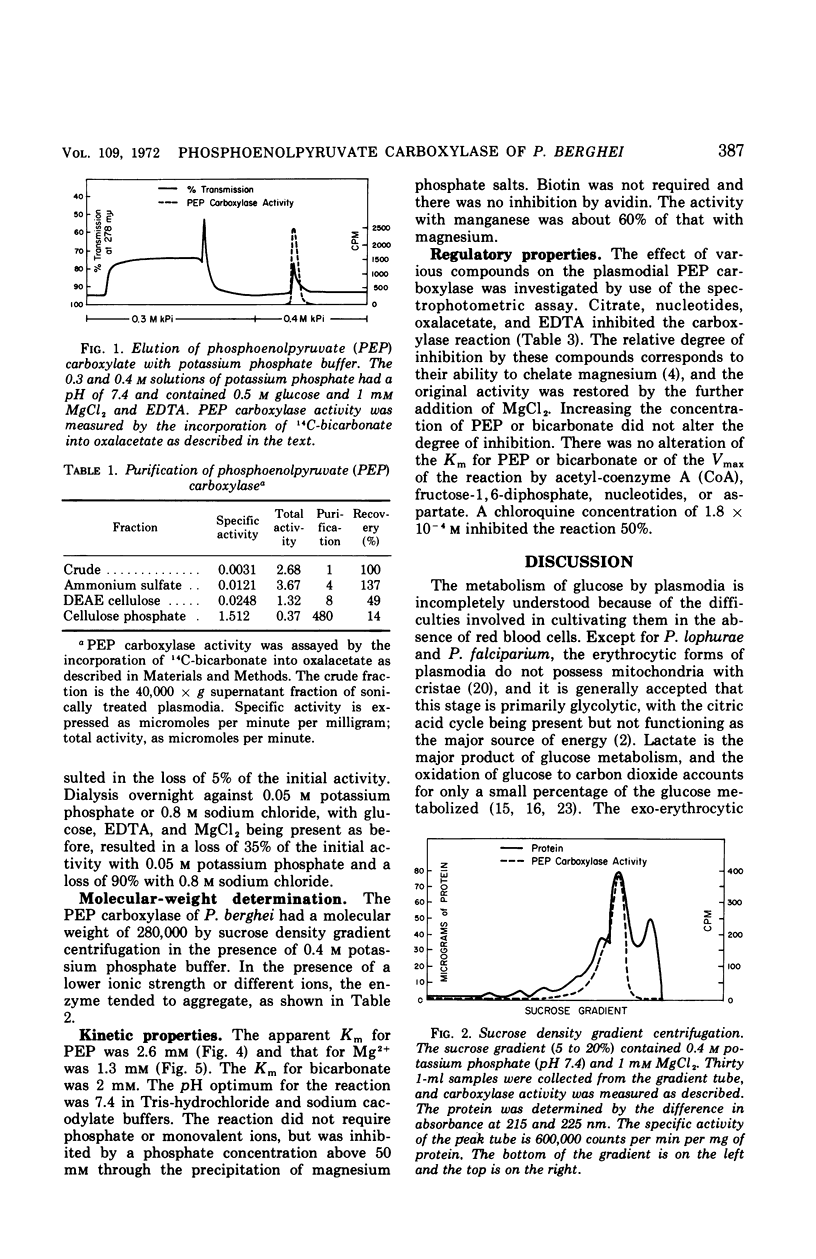
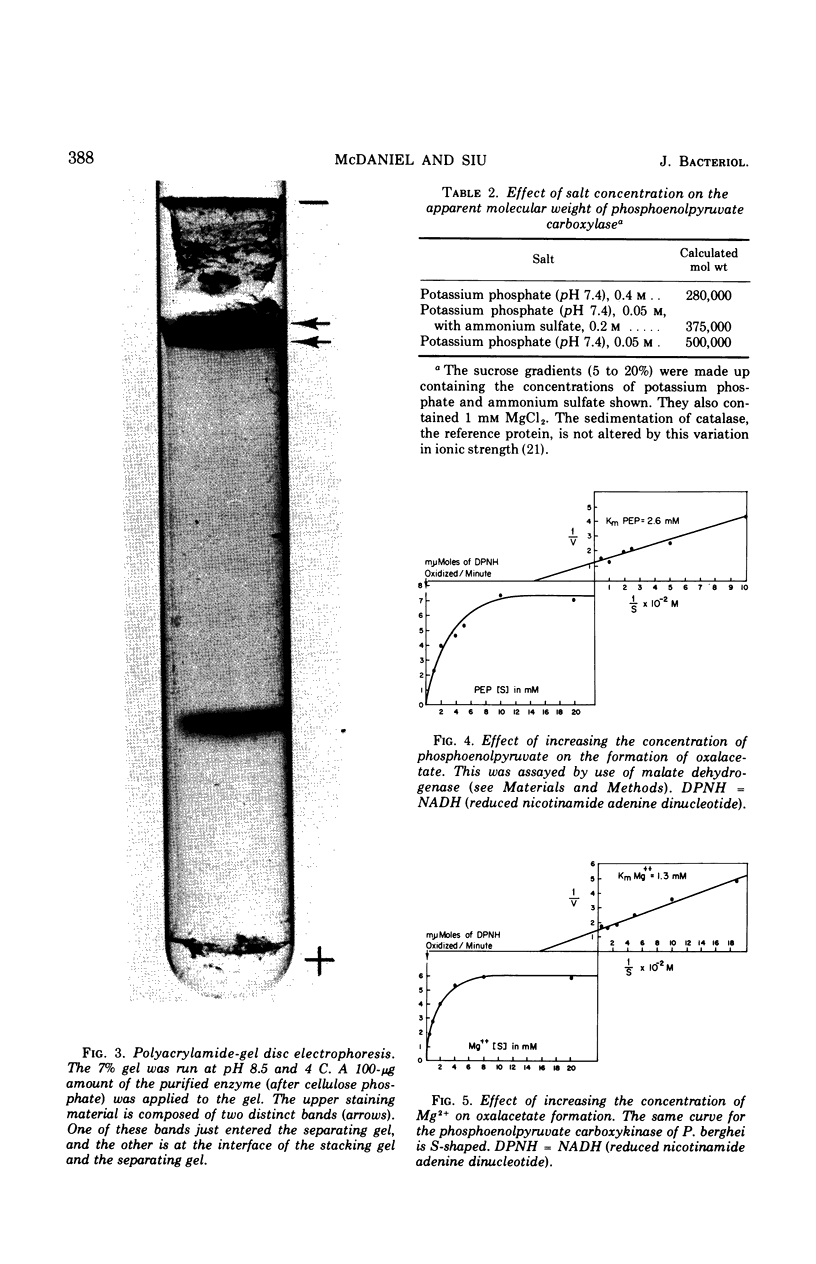


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN M., HUGHES T. R., Jr Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J Biol Chem. 1962 Jan;237:176–181. [PubMed] [Google Scholar]
- Cohen S. N., Yielding K. L. Inhibition of DNA and RNA polymerase reactions by chloroquine. Proc Natl Acad Sci U S A. 1965 Aug;54(2):521–527. doi: 10.1073/pnas.54.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R. Studies of parameters affecting the allosteric nature of phosphoenolpyruvate carboxylase of Escherichia coli. J Biol Chem. 1968 Jun 25;243(12):3517–3525. [PubMed] [Google Scholar]
- Fitch C. D. Chloroquine resistance in malaria: a deficiency of chloroquine binding. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1181–1187. doi: 10.1073/pnas.64.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Howells R. E. Cytochrome oxidase activity in a normal and some drug-resistant strains of Plasmodium berghei--a cytochemical study. II. Sporogonic stages of a drug-sensitive srain. Ann Trop Med Parasitol. 1970 Jun;64(2):223–225. doi: 10.1080/00034983.1970.11686684. [DOI] [PubMed] [Google Scholar]
- Howells R. E., Peters W., Homewood C. A., Warhurst D. C. Theory for the mechanism of chloroquine resistance in rodent malaria. Nature. 1970 Nov 14;228(5272):625–628. doi: 10.1038/228625a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. Phosphoenolpyruvate carboxylase of Salmonella. Some chemical and allosteric properties. J Biol Chem. 1969 May 25;244(10):2549–2557. [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- Nagarajan K. Metabolism of Plasmodium berghei. I. Krebs cycle. Exp Parasitol. 1968 Feb;22(1):19–26. doi: 10.1016/0014-4894(68)90074-x. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Polet H., Conrad M. E. In vitro studies on the amino acid metabolism of Plasmodium knowlesi and the antiplasmodial effect of the isoleucine antagonists. Mil Med. 1969 Sep;134(10):939–944. [PubMed] [Google Scholar]
- Polet H., Conrad M. E. Malaria: extracellular amino acid requirements for in vitro growth of erythrocytic forms of Plasmodium knowlesi. Proc Soc Exp Biol Med. 1968 Jan;127(1):251–253. doi: 10.3181/00379727-127-32666. [DOI] [PubMed] [Google Scholar]
- Rudzinska M. A., Trager W., Bray R. S. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J Protozool. 1965 Nov;12(4):563–576. doi: 10.1111/j.1550-7408.1965.tb03256.x. [DOI] [PubMed] [Google Scholar]
- SAMEJIMA T., KAMATA M., SHIBATA K. Dissociation of bovine liver catalase at low pH. J Biochem. 1962 Mar;51:181–187. doi: 10.1093/oxfordjournals.jbchem.a127518. [DOI] [PubMed] [Google Scholar]
- SANADI D. R., FLUHARTY A. L. ON THE MECHANISM OF OXIDATIVE PHOSPHORYLATION. VII. THE ENERGY-REQUIRING REDUCTION OF PYRIDINE NUCLEOTIDE BY SUCCINATE AND THE ENERGY-YIELDING OXIDATION OF REDUCED PYRIDINE NUCLEOTIDE BY FUMARATE. Biochemistry. 1963 May-Jun;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Miller J. Glycolytic and cytochrome oxidase activity in Plasmodia. Mil Med. 1969 Sep;134(10):1074–1080. [PubMed] [Google Scholar]
- Sherman I. W., Ruble J. A., Ting I. P. Plasmodium lophurae: (U-14C)-glucose catabolism by free Plasmodia and duckling host erythrocytes. Exp Parasitol. 1969 Aug;25(1):181–192. doi: 10.1016/0014-4894(69)90064-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Schnell J. V., Geiman Q. M. Nutritional requirements for in vitro cultivation of a simian malarial parasite, Plasmodium knowlesi. Mil Med. 1969 Sep;134(10):929–938. [PubMed] [Google Scholar]
- Siu P. M. Carbon dioxide fixation in plasmodia and the effect of some antimalarial drugs on the enzyme. Comp Biochem Physiol. 1967 Dec;23(3):785–795. doi: 10.1016/0010-406x(67)90341-6. [DOI] [PubMed] [Google Scholar]
- Skelton F. S., Pardini R. S., Heidker J. C., Folkers K. Inhibition of coenzyme Q systems by chloroquine and other antimalarials. J Am Chem Soc. 1968 Sep 11;90(19):5334–5336. doi: 10.1021/ja01021a084. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., VENNESLAND B. Enzymatic carbon dioxide fixation into oxal-acetate in wheat germ. J Biol Chem. 1955 Apr;213(2):533–546. [PubMed] [Google Scholar]
- TRAGER W. CULTIVATION AND PHYSIOLOGY OF ERYTHROCYTIC STAGES OF PLASMODIA. Am J Trop Med Hyg. 1964 Jan;13:SUPPL–166. doi: 10.4269/ajtmh.1964.13.162. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]



