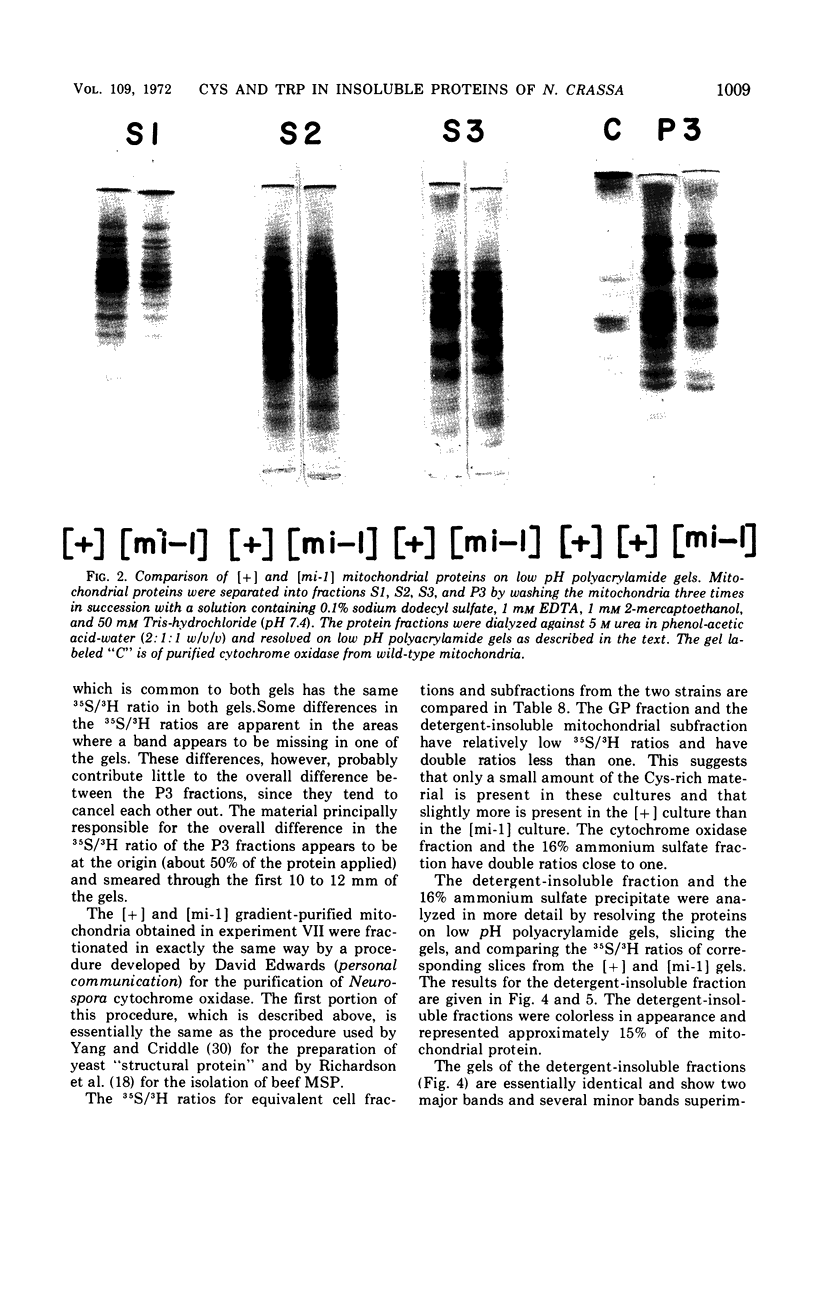
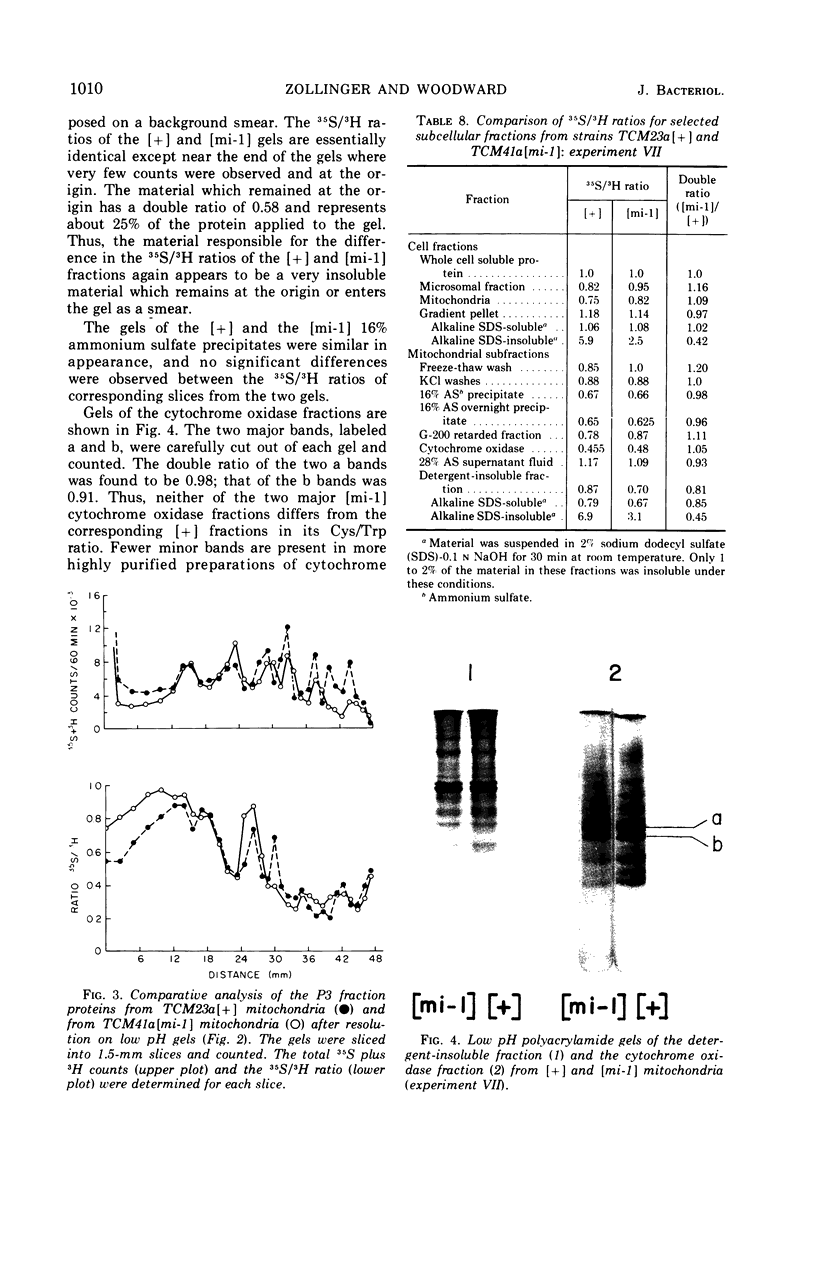
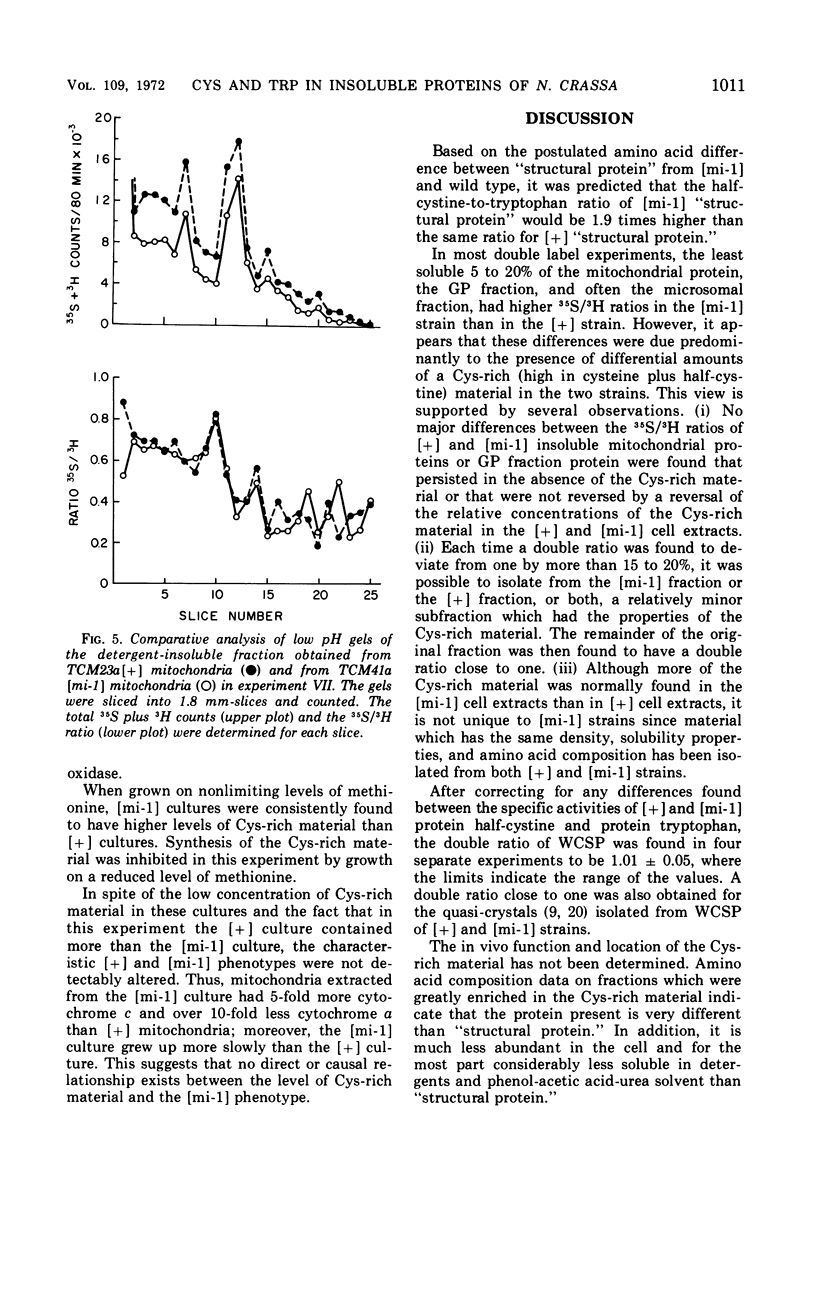
Abstract
The possibility of an amino acid substitution (cysteine for tryptophan) in a membrane protein of the [mi-1] strain of Neurospora crassa has been investigated in detail by using a double radioactive labeling procedure. Auxotrophic strains of Neurospora having wild-type [+] or [mi-1] cytoplasm have been grown under conditions which result in the specific labeling of protein tryptophan with 3H and protein cysteine with 35S. Although the least soluble 1 to 20% of the [mi-1] mitochondrial membrane protein was usually found to have a higher Cys/Trp ratio (ratio of cysteine plus half-cystine to tryptophan) than the corresponding [+] fraction, it has been shown that these differences were due mainly to the presence of differential amounts of a very insoluble, cysteine-rich (Cys-rich) material. The same Cys-rich material was found in variable amounts in both [+] and [mi-1] cultures, but the concentration was usually higher in the [mi-1] cultures. The Cys-rich material is clearly distinct from “structural protein” on the basis of amino acid composition and appears to have no direct relationship to the [mi-1] phenotype. In the absence of the Cys-rich material, no difference between the Cys/Trp ratios of corresponding [+] and [mi-1] membrane proteins could be detected. We conclude, therefore, that the previously postulated amino acid substitution of cysteine for tryptophan in [mi-1] membrane protein is incorrect.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- Birkmayer G. D., Bücher T. Ferrochelatase in wild-type and in cytoplasmic mutants of neurospora crassa. FEBS Lett. 1969 Sep;5(1):28–30. doi: 10.1016/0014-5793(69)80284-x. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Mahler H. R. Resolution of insoluble proteins in rat brain subcellular fractions. Arch Biochem Biophys. 1967 May;120(2):384–396. doi: 10.1016/0003-9861(67)90255-x. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Woodward D. O. An altered cytochrome oxidase in a cytoplasmic mutant of Neurospora. FEBS Lett. 1969 Aug;4(3):193–196. doi: 10.1016/0014-5793(69)80232-2. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The regulation of synthesis of Krebs cycle enzymes in Neurospora by catabolite and end product repression. Eur J Biochem. 1970 Apr;13(3):548–553. doi: 10.1111/j.1432-1033.1970.tb00959.x. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- HASKINS F. A., TISSIERES A., MITCHELL H. K., MITCHELL M. B. Cytochromes and the succinic acid oxidase system of poky strains of Neurospora. J Biol Chem. 1953 Feb;200(2):819–826. [PubMed] [Google Scholar]
- Kaplan D. M., Criddle R. S. Membrane structural proteins. Physiol Rev. 1971 Apr;51(2):249–272. doi: 10.1152/physrev.1971.51.2.249. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A., Johanson R. A., Hill J. M., Shumway L. K. The facile isolation of a structural phospholipoprotein from hydrogenomon as facilis and Neurospora crassa. Proc Natl Acad Sci U S A. 1969 Feb;62(2):407–414. doi: 10.1073/pnas.62.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G., Haard N. F., Lauwers A., Allmann D. W., Green D. E. Mitochondrial structural protein I. Methods of preparation and purification: characterization by gel electrophoresis. Arch Biochem Biophys. 1968 Sep 10;126(3):746–772. doi: 10.1016/0003-9861(68)90466-9. [DOI] [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K. A Case of "Maternal" Inheritance in Neurospora Crassa. Proc Natl Acad Sci U S A. 1952 May;38(5):442–449. doi: 10.1073/pnas.38.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W. M. A potential source of electrophoretic artifacts in polyacrylamide gels. Biochim Biophys Acta. 1967 Sep 19;147(1):171–174. doi: 10.1016/0005-2795(67)90101-8. [DOI] [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., MORINO Y., SNELL E. E. PROPERTIES OF CRYSTALLINE TRYPTOPHANASE. J Biol Chem. 1965 Mar;240:1211–1218. [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. An inducible tryptophan synthetase in tryptophan auxotrophs of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1431–1439. doi: 10.1073/pnas.48.8.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLTMANN E. A., MAHOWALD T. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. II. Amino acid composition of adenosine triphosphate-creatine transphosphorylase. J Biol Chem. 1962 Apr;237:1146–1154. [PubMed] [Google Scholar]
- RICHARDSON S. H., HULTIN H. O., FLEISCHER S. INTERACTIONS OF MITOCHONDRIAL STRUCTURAL PROTEIN WITH PHOSPHOLIPIDS. Arch Biochem Biophys. 1964 May;105:254–260. doi: 10.1016/0003-9861(64)90006-2. [DOI] [PubMed] [Google Scholar]
- Sebald W., Bücher T., Olbrich B., Kaudewitz F. Electrophoretic pattern of and amino acid incorporation in vitro into the insoluble mitochondrial protein of neurospora crassa wild type and mi-1 mutant. FEBS Lett. 1968 Sep;1(4):235–240. doi: 10.1016/0014-5793(68)80071-7. [DOI] [PubMed] [Google Scholar]
- Shannon C. F., Hill J. M. A cytoplasmic protein from Neurospora crassa resembling membrane proteins. Biochemistry. 1971 Aug 3;10(16):3021–3029. doi: 10.1021/bi00792a006. [DOI] [PubMed] [Google Scholar]
- TISSIERES A., MITCHELL H. K., HASKINS F. A. Studies on the respiratory system of the poky strain of Neurospora. J Biol Chem. 1953 Nov;205(1):423–433. [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woodward D. O. Functional and organizational properties of Neurospora mitochondrial structural protein. Fed Proc. 1968 Sep-Oct;27(5):1167–1173. [PubMed] [Google Scholar]
- Woodward D. O., Munkres K. D. Alterations of a maternally inherited mitochondrial structural protein in respiratory-deficient strains of Neurospora. Proc Natl Acad Sci U S A. 1966 Apr;55(4):872–880. doi: 10.1073/pnas.55.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Criddle R. S. Identification of a major membrane fraction as a product of synthesis by isolated yeast mitochondria. Biochem Biophys Res Commun. 1969 May 22;35(4):429–436. doi: 10.1016/0006-291x(69)90363-5. [DOI] [PubMed] [Google Scholar]