Abstract
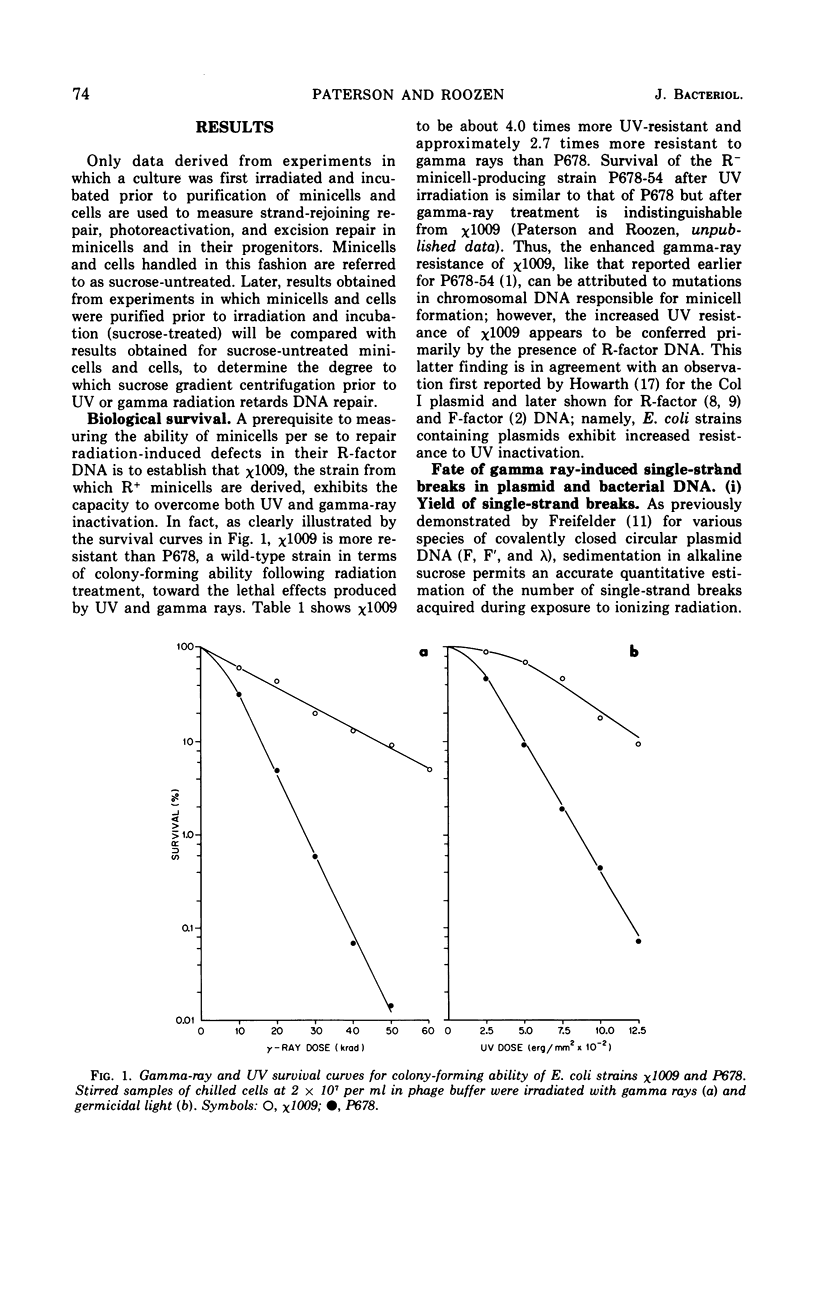
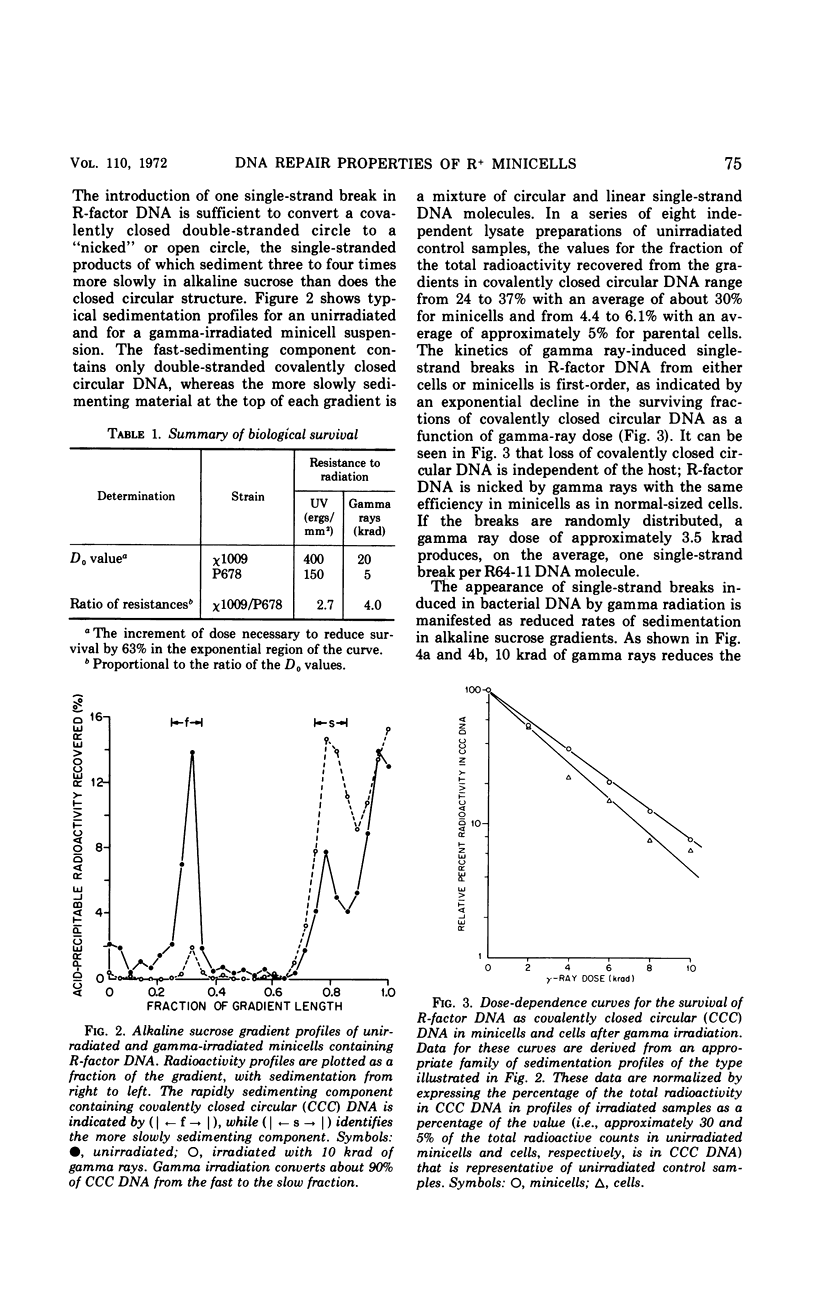
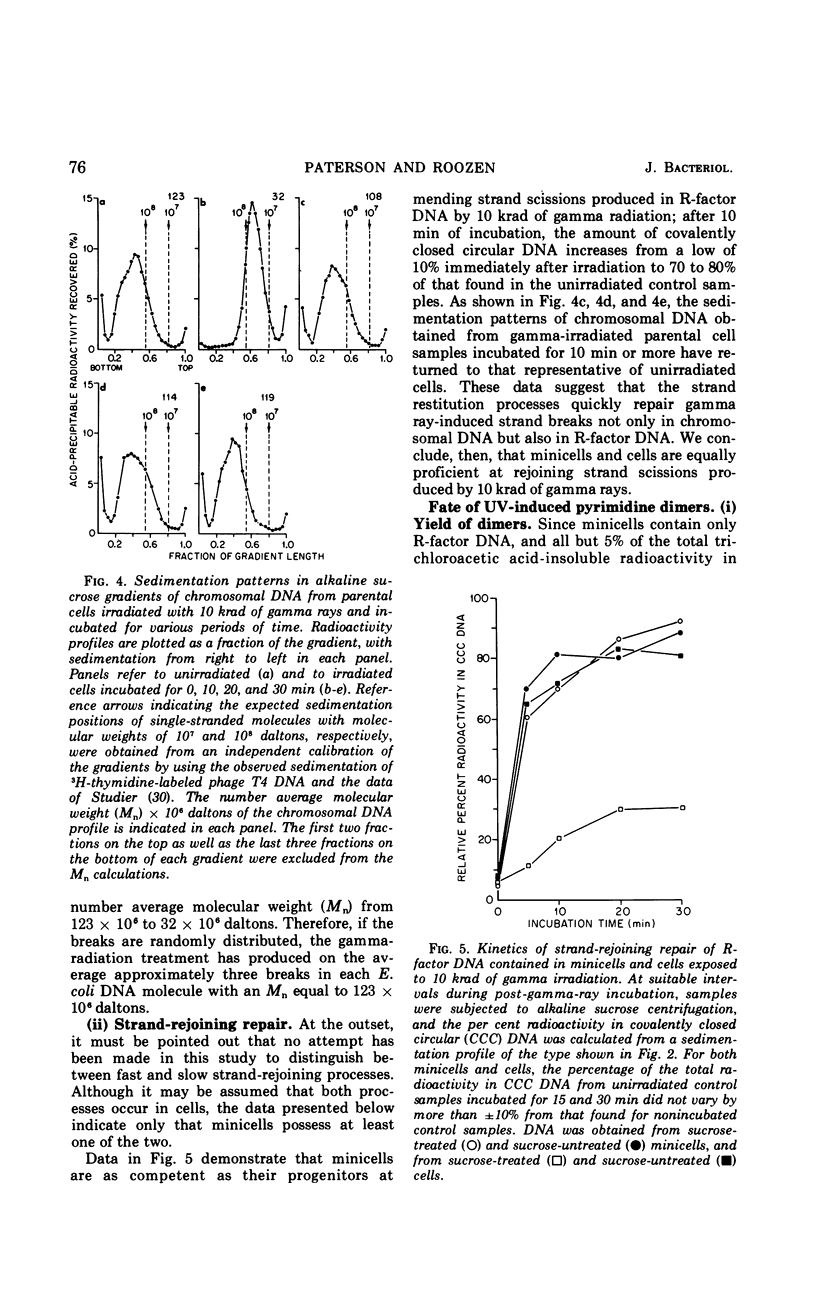
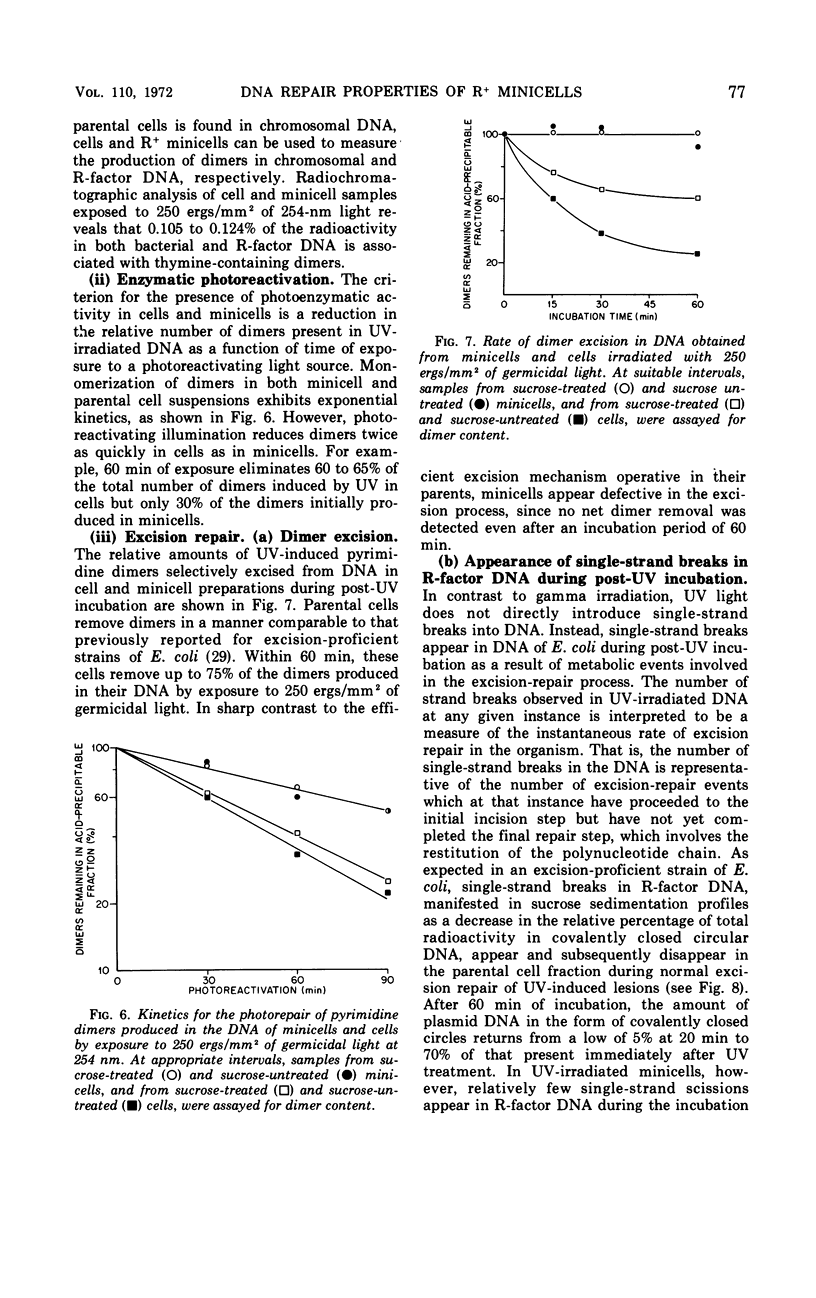
Chromosomeless “minicells” are formed by misplaced cell fissions near the polar extremities of an Escherichia coli K-12 mutant strain. Resistance (R)-factor deoxyribonucleic acid (DNA) can be introduced into minicells by segregation from an R+ (R64-11) derivative of the original mutant. We have assessed the ability of R+ minicells to correct defects produced in their plasmid DNA by ultraviolet (UV) and gamma radiations. Minicells harboring plasmid DNA, in comparison with their repair-proficient minicell-producing parents, possess (i) an equal competence to rejoin single-strand breaks induced in DNA by gamma rays, (ii) a reduced capacity for the photoenzymatic repair of UV-induced pyrimidine dimers, and (iii) a total inability to excise dimers, apparently owing to a deficiency in UV-specific endonuclease activity responsible for mediating the initial incision step in excision repair. Assuming that the DNA repair properties of R+ minicells reflect the concentration of repair enzymes located in the plasmid-containing polar caps of entire cells, these findings suggest that: (i) the enzymes responsible for rejoining single-strand breaks are distributed throughout the cell; (ii) photoreactivating enzyme molecules tend to be concentrated near bacterial DNA and to a lesser extent near plasmid DNA; and (iii) UV-specific endonuclease molecules are primarily confined to the central region of the E. coli cell and, thus, seldom segregate with R-factor DNA into minicells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. E., Adler H. I. Influence of the fertility episome on the survival of x-irradiated Escherichia coli. J Bacteriol. 1969 Apr;98(1):329–330. doi: 10.1128/jb.98.1.329-330.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Fraser D., Mahler H. R., Shug A. L., Thomas C. A. THE INFECTION OF SUB-CELLULAR ESCHERICHIA COLI, STRAIN B, WITH A DNA PREPARATION FROM T2 BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1957 Nov 15;43(11):939–947. doi: 10.1073/pnas.43.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Rate of production of single-strand breaks in DNA by x-irradiation in situ. J Mol Biol. 1968 Jul 28;35(2):303–309. doi: 10.1016/s0022-2836(68)80026-9. [DOI] [PubMed] [Google Scholar]
- Grossman L., Kaplan J. C., Kushner S. R., Mahler I. Enzymes involved in the early stages of repair of ultraviolet-irradiated DNA. Cold Spring Harb Symp Quant Biol. 1968;33:229–234. doi: 10.1101/sqb.1968.033.01.026. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Howarth S. Resistance to the bactericidal effect of ultraviolet radiation conferred on Enterobacteria by the colicine factor coli. J Gen Microbiol. 1965 Jul;40(1):43–55. doi: 10.1099/00221287-40-1-43. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


