Abstract
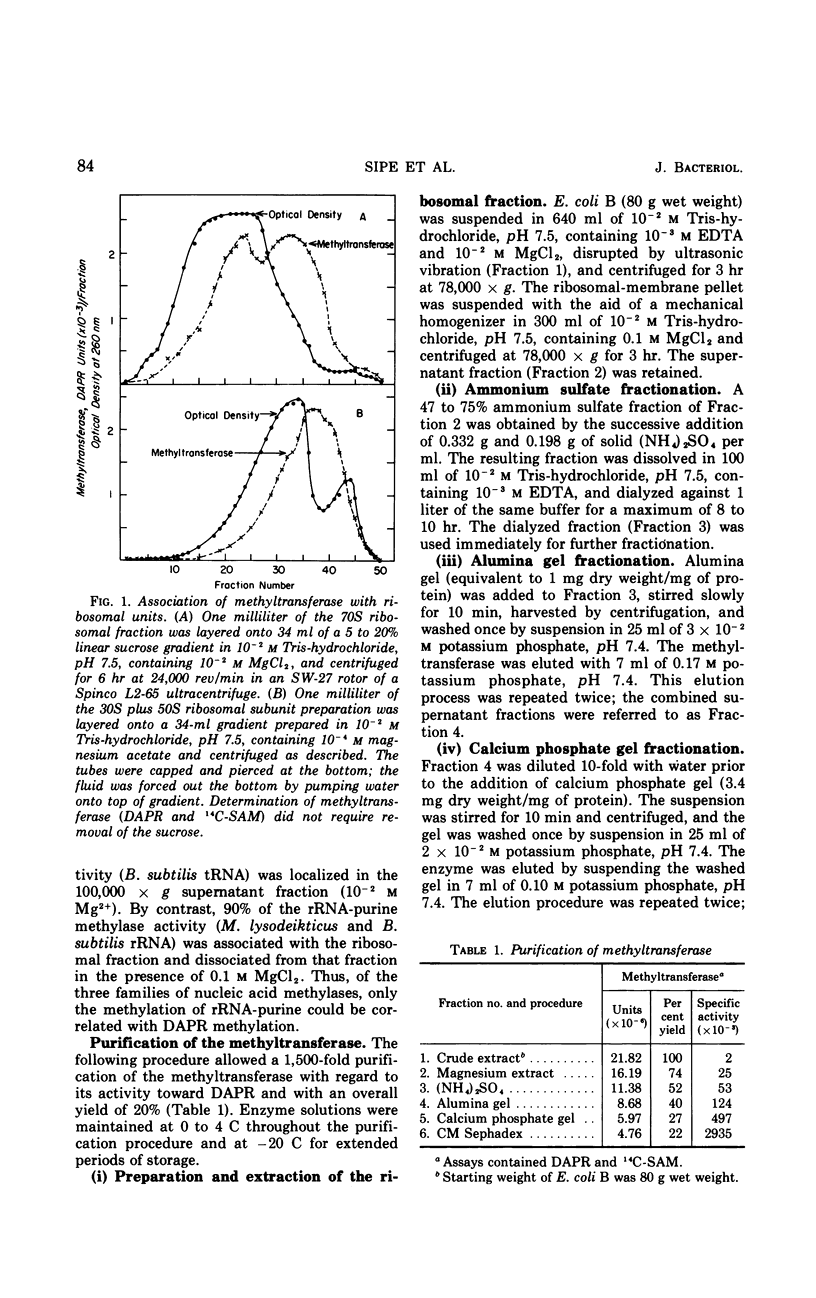
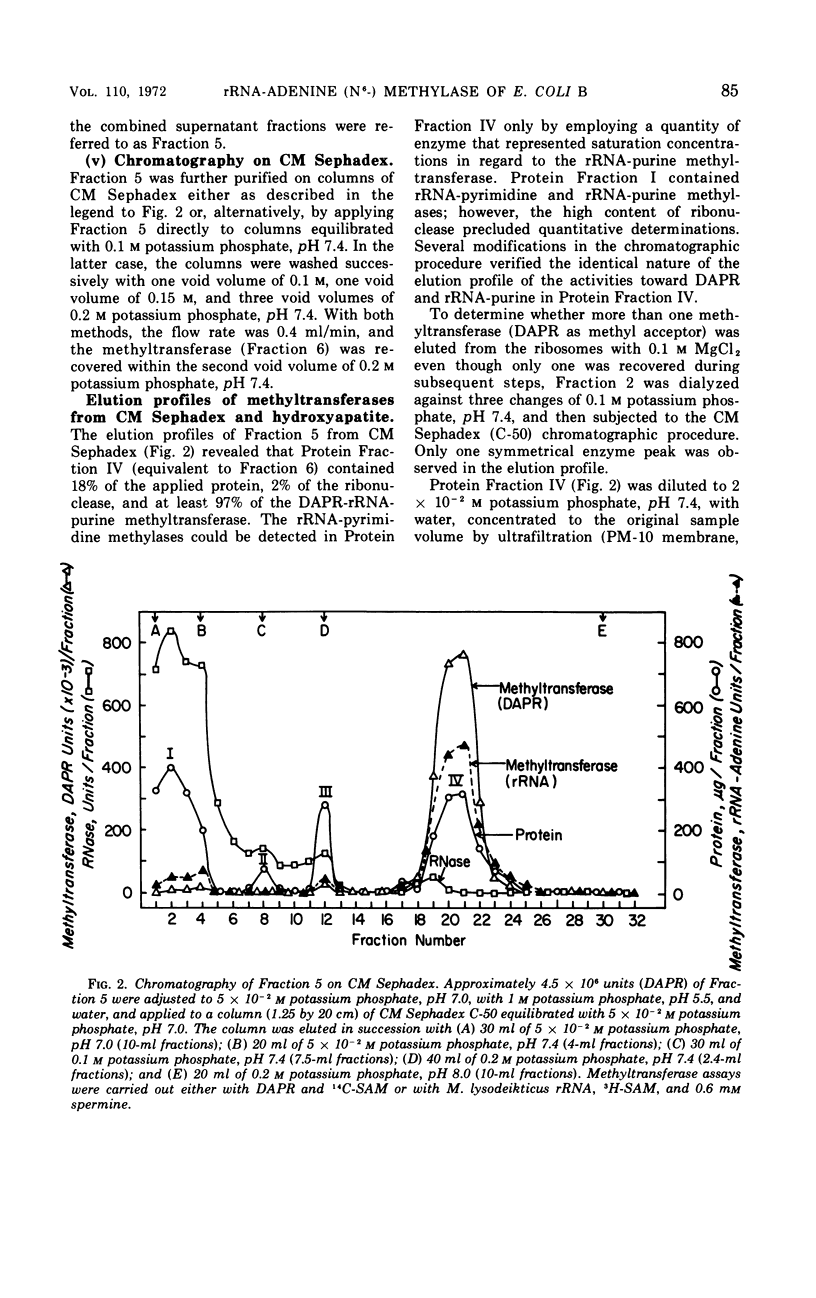
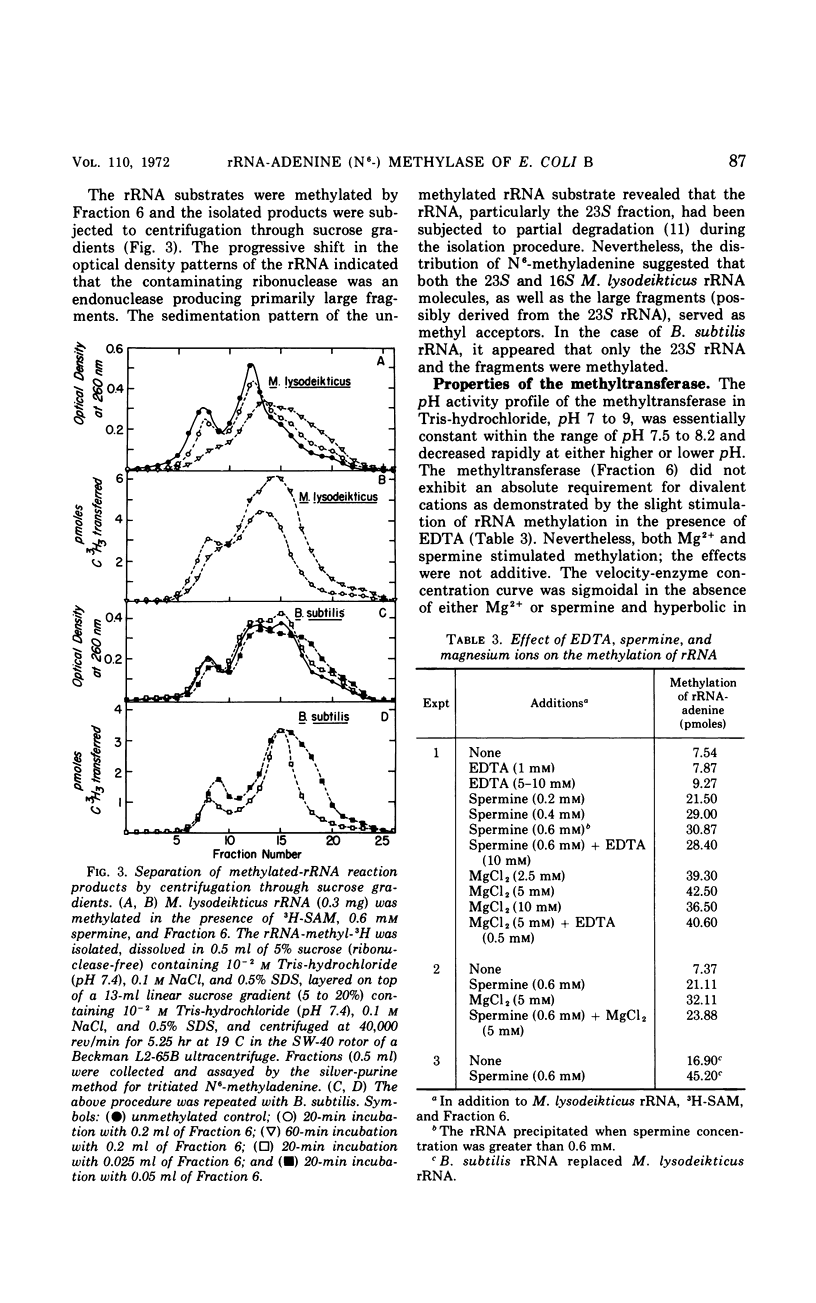
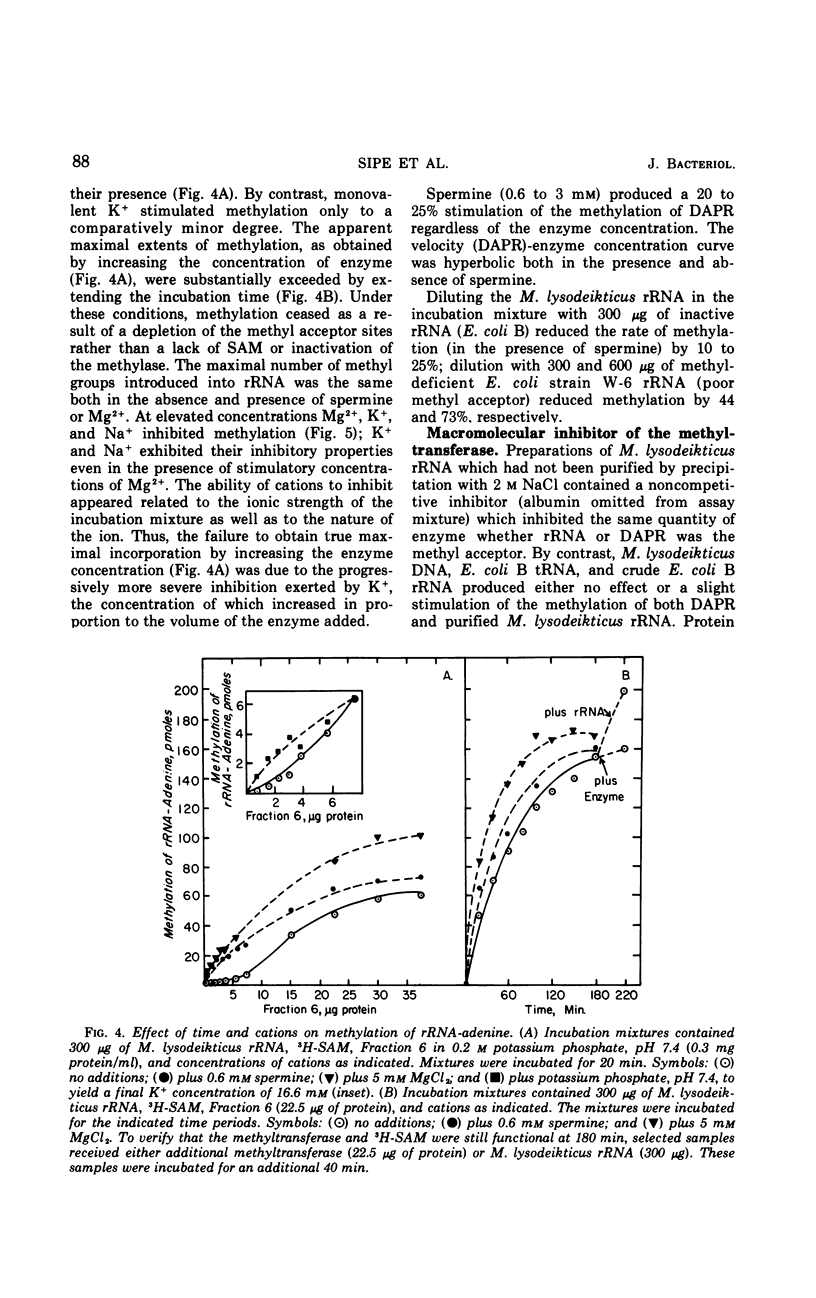
This study is concerned with the isolation and characterization of the enzyme, S-adenosylmethionine:ribosomal ribonucleic acid-adenine (N6−) methyl-transferase [rRNA-adenine (N6-) methylase] of Escherichia coli strain B, which is responsible for the formation of N6-methyladenine moieties in ribosomal ribonucleic acids (rRNA). A 1,500-fold purified preparation of the species-specific methyltransferase methylates a limited number of adenine moieties in heterologous rRNA (Micrococcus lysodeikticus and Bacillus subtilis) and methyl-deficient homologous rRNA. The site recognition mechanism does not require intact 16 or 23S rRNA. The enzyme does not utilize transfer ribonucleic acid as a methyl acceptor nor does it synthesize 2-methyladenine or N6-dimethyladenine moieties. Mg2+, spermine, K+, and Na+ increase the reaction rate but not the extent of methylation; elevated concentrations of the cations inhibit markedly. The purified preparations utilize 9-β-ribosyl-2,6-diaminopurine (DAPR) as a methyl acceptor with the synthesis of 9-β-ribosyl-6-amino-2-methylaminopurine. A comparison of the two activities demonstrated that one methyltransferase is responsible for the methylation of both DAPR and rRNA. This property provides a sensitive assay procedure unaffected by ribonucleases and independent of any specificity exhibited by rRNA methyl acceptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S. S., ARBOGAST R. Chemical studies in host-virus interactions; a comparison of some properties of three mutant pairs of bacterial viruses, T2r and T2r, T4r and T4r, T6r and T6r. J Exp Med. 1950 Jun 1;91(6):619–636. doi: 10.1084/jem.91.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Carr C. W. Ion-binding studies of ribonucleic acid and Escherichia coli ribosomes. J Mol Biol. 1967 Apr 28;25(2):331–345. doi: 10.1016/0022-2836(67)90145-3. [DOI] [PubMed] [Google Scholar]
- Fellner P., Sanger F. Sequence analysis of specific areas of the 16S and 23S ribosomal RNAs. Nature. 1968 Jul 20;219(5151):236–238. doi: 10.1038/219236a0. [DOI] [PubMed] [Google Scholar]
- GORDON J., BOMAN H. G. STUDIES ON MICROBIAL RNA.II. TRANSFER OF METHYL GROUPS FROM METHIONINE TO THE RNA OF A RIBONUCLEOPROTEIN PARTICLE. J Mol Biol. 1964 Sep;9:638–653. doi: 10.1016/s0022-2836(64)80172-8. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., ANDERS M., GOLD M., SMITH I. THE ENZYMATIC METHYLATION OF RIBONUCLEIC ACID AND DEOXYRIBONUCLEIC ACID. VII. THE METHYLATION OF RIBONUCLEIC ACID. J Biol Chem. 1965 Mar;240:1256–1266. [PubMed] [Google Scholar]
- HURWITZ J., GOLD M., ANDERS M. THE ENZYMATIC METHYLATION OF RIBONUCLEIC ACID AND DEOXYRIBONUCLEIC ACID. 3. PURIFICATION OF SOLUBLE RIBONUCLEIC ACID-METHYLATING ENZYMES. J Biol Chem. 1964 Oct;239:3462–3473. [PubMed] [Google Scholar]
- Isaksson L. A., Phillips J. H. Studies on microbial RNA. V. A comparison of the in vivo methylated components of ribosomal RNA from Escherichia coli and Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Jan 29;155(1):63–71. [PubMed] [Google Scholar]
- Leboy P. S. Stimulation of soluble ribonucleic acid methylase activity by polyamines. Biochemistry. 1970 Mar 31;9(7):1577–1584. doi: 10.1021/bi00809a016. [DOI] [PubMed] [Google Scholar]
- MIDGLEY J. E. EFFECTS OF DIFFERENT EXTRACTION PROCEDURES ON THE MOLECULAR CHARACTERISTICS OF BACTERIAL RIBOSOMAL RIBONUCLEIC ACID. Biochim Biophys Acta. 1965 Feb 8;95:232–243. doi: 10.1016/0005-2787(65)90488-0. [DOI] [PubMed] [Google Scholar]
- McIlreavy D. J., Midgley J. E. The chemical structure of bacterial ribosomal RNA. I. Terminal nucleotide sequences of Escherichia coli ribosomal RNA. Biochim Biophys Acta. 1967 Jun 20;142(1):47–64. doi: 10.1016/0005-2787(67)90514-x. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. SOME OBSERVATIONS ON THE "LATENT" RIBONUCLEASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Jun;51:1267–1274. doi: 10.1073/pnas.51.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. L., Lane B. G. In vitro O2'-methylation of sugars in E. coli RNA. II. Methylation of ribosomal and transfer RNA by homologous methylases in crude cell-free extracts and particulate suspensions from a relaxed mutant of E. coli. Can J Biochem. 1968 Dec;46(12):1487–1495. doi: 10.1139/o68-222. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. The effects of diamines and polyamines on enzymic methylation of nucleic acid. Biochim Biophys Acta. 1971 Apr 8;232(4):630–642. doi: 10.1016/0005-2787(71)90755-6. [DOI] [PubMed] [Google Scholar]
- REMY C. N. Metabolism of 2,6-diaminopurine: S-adenosylmethionine as methyl donor for 2-methylamino-6-aminopurine synthesis. J Biol Chem. 1959 Jun;234(6):1485–1491. [PubMed] [Google Scholar]
- REMY C. N., SMITH M. S. Metabolism of 2, 6-diaminopurine; conversion to 5'-phosphoribosyl-2-methylamino-beta-aminopurine by enzymes of Escherichia coli. J Biol Chem. 1957 Sep;228(1):325–338. [PubMed] [Google Scholar]
- Remy C. N. Ribonucleotides and ribonucleosides as methyl acceptors for S-adenosylmethionine: (amino- and thio-)purine methyl-transferases. Incorporation of 6-amino-2-methylaminopurine into ribonucleic acids. Biochim Biophys Acta. 1967 Apr 18;138(2):258–275. [PubMed] [Google Scholar]
- SUZUKI H., HAYASHI Y. THE FORMATION OF "RIBOSOMAL RNA" IN ESCHERICHIA COLI DURING RECOVERY FROM MAGNESIUM STARVATION. Biochim Biophys Acta. 1964 Aug 12;87:610–620. doi: 10.1016/0926-6550(64)90279-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. F. Secondary methylation of ribosomal ribonucleic acid in HeLa cells. Biochemistry. 1968 Sep;7(9):3156–3164. doi: 10.1021/bi00849a019. [DOI] [PubMed] [Google Scholar]


