Abstract
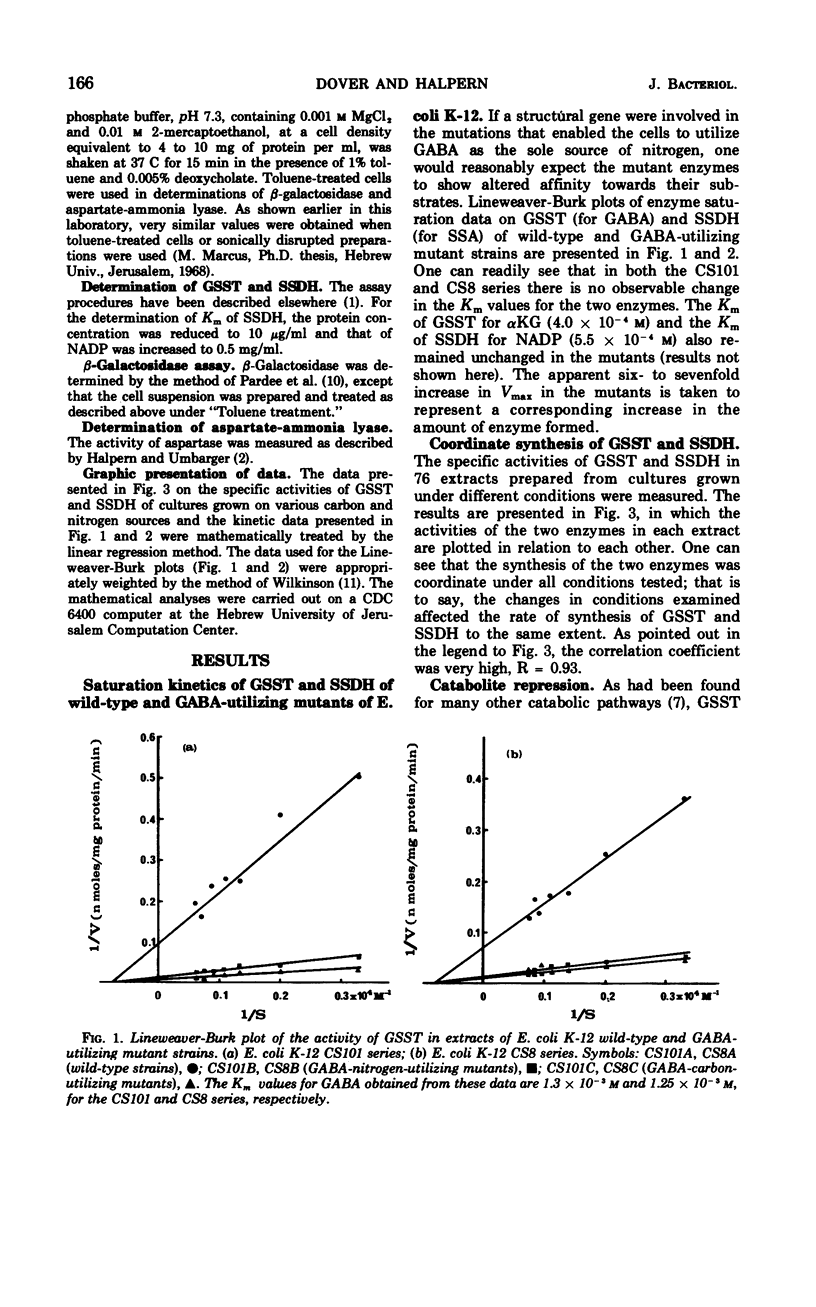
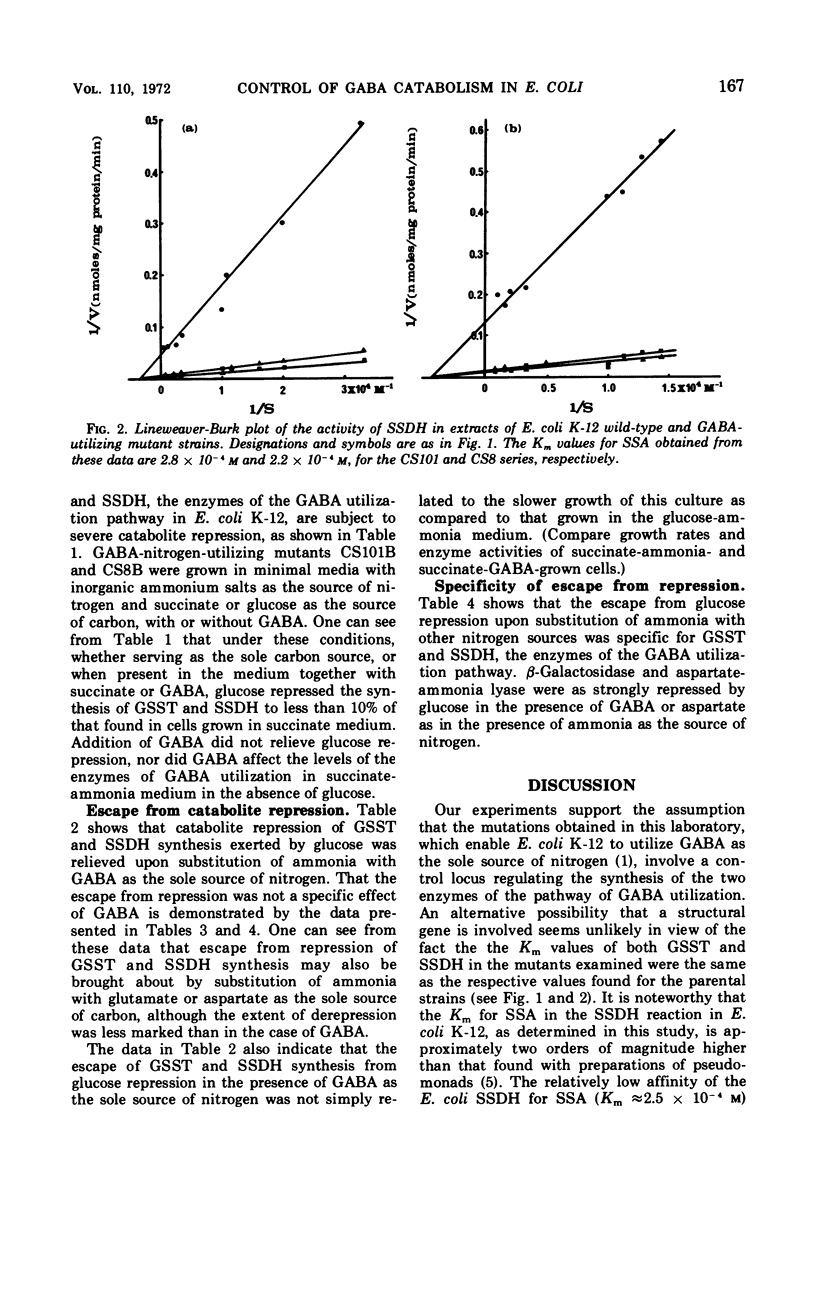
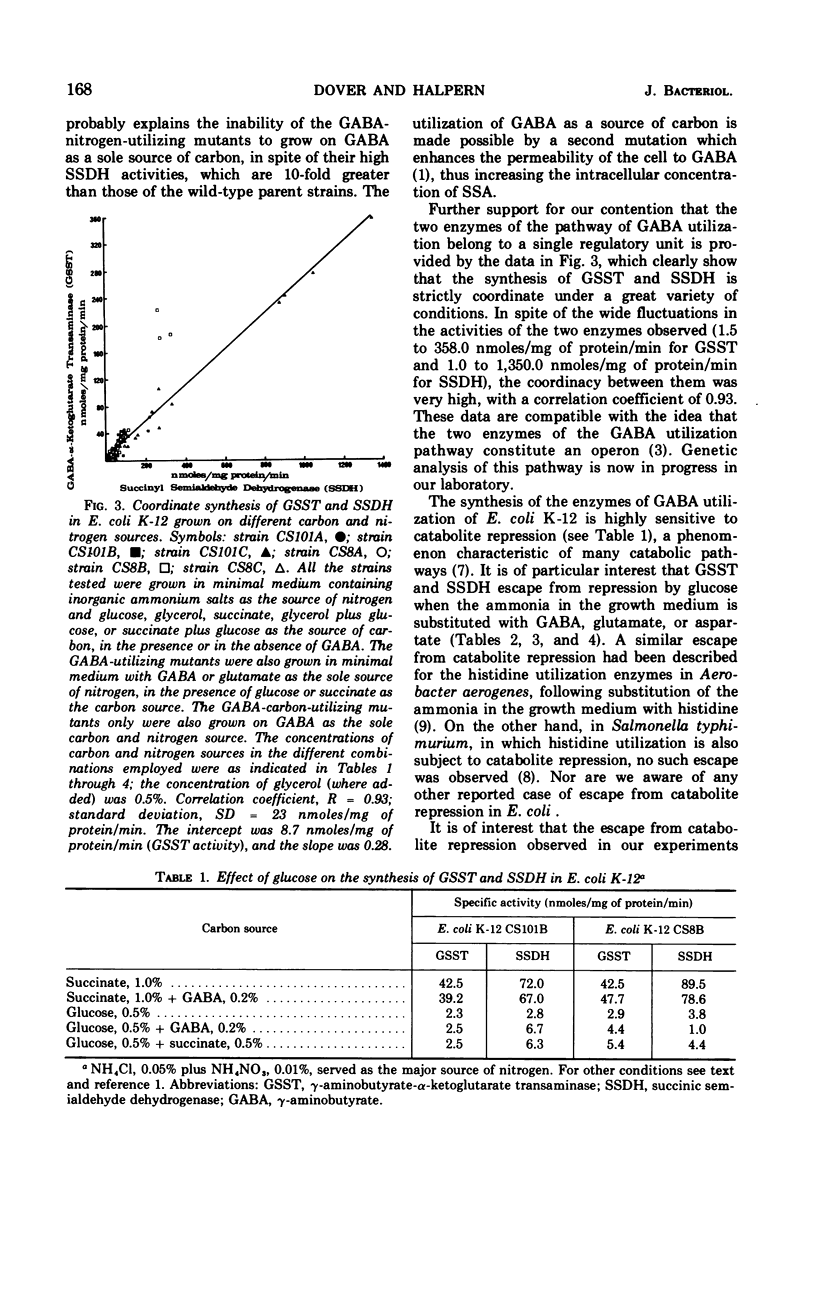
Mutants of Escherichia coli K-12 isolated for their ability to utilize γ-aminobutyrate (GABA) as the sole source of nitrogen exhibit a concomitant several-fold increase in the activities of γ-aminobutyrate-α-ketoglutarate transaminase (GSST, EC 2.6.1.19) and succinic semialdehyde dehydrogenase (SSDH, EC 1.2.1.16). The increase in rate of enzymatic activity is not accompanied by any changes in the affinities of the mutant enzymes for their respective substrates. The synthesis of the two enzymes is highly coordinate under a great variety of conditions, in spite of the wide range of activities observed. In cultures grown in minimal media with ammonium salts as the source of nitrogen, both GSST and SSDH are severely repressed by glucose. Substitution of ammonia with GABA, glutamate, or aspartate greatly reduces the effect of glucose on the synthesis of the GABA utilization enzymes. This escape from catabolite repression is specific for GSST and SSDH and does not involve other enzymes sensitive to catabolite repression (e.g., β-galactosidase, EC 3.2.1.23, and aspartase, EC 4.3.1.1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dover S., Halpern Y. S. Utilization of -aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol. 1972 Feb;109(2):835–843. doi: 10.1128/jb.109.2.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B., SCOTT E. M. Aldehyde oxidation. III. Succinic semialdehyde dehydrogenase. J Biol Chem. 1959 Apr;234(4):937–940. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


