Abstract
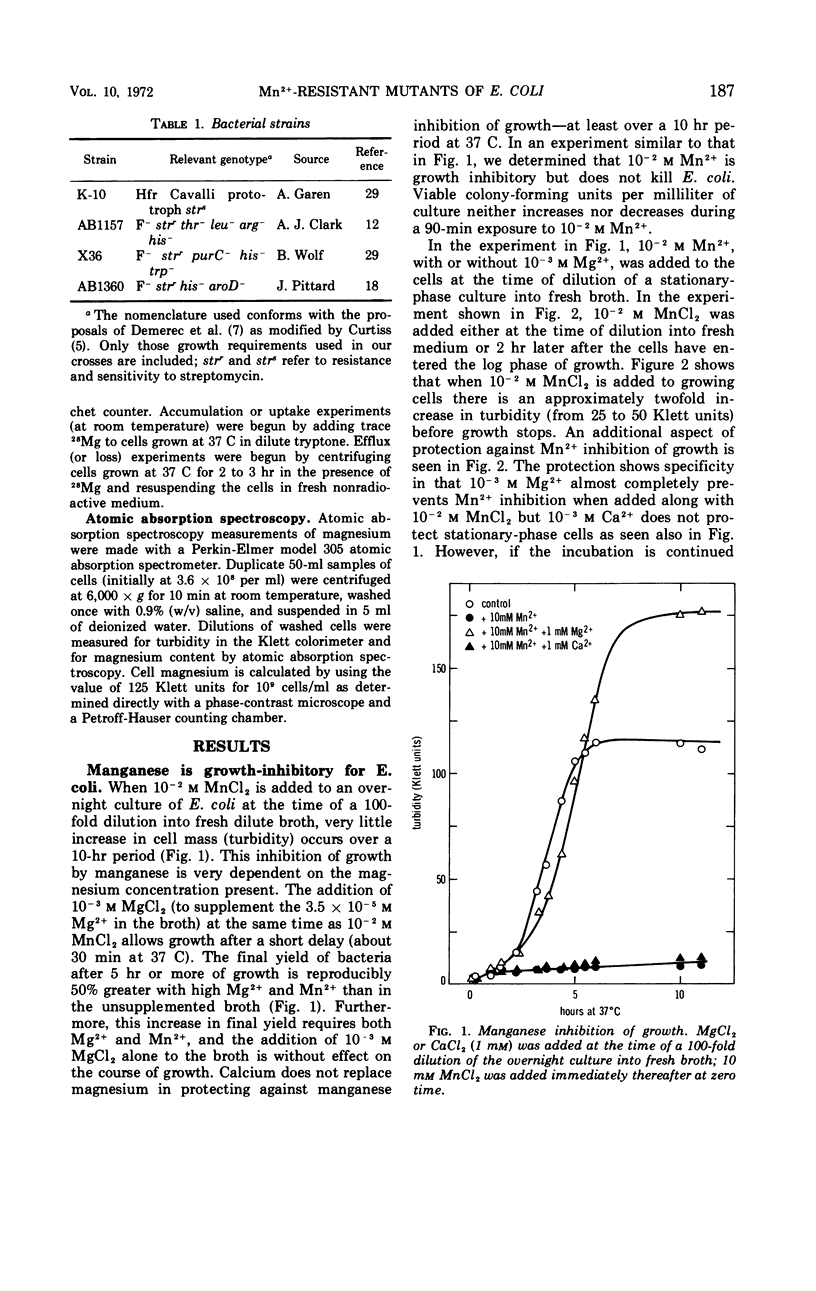
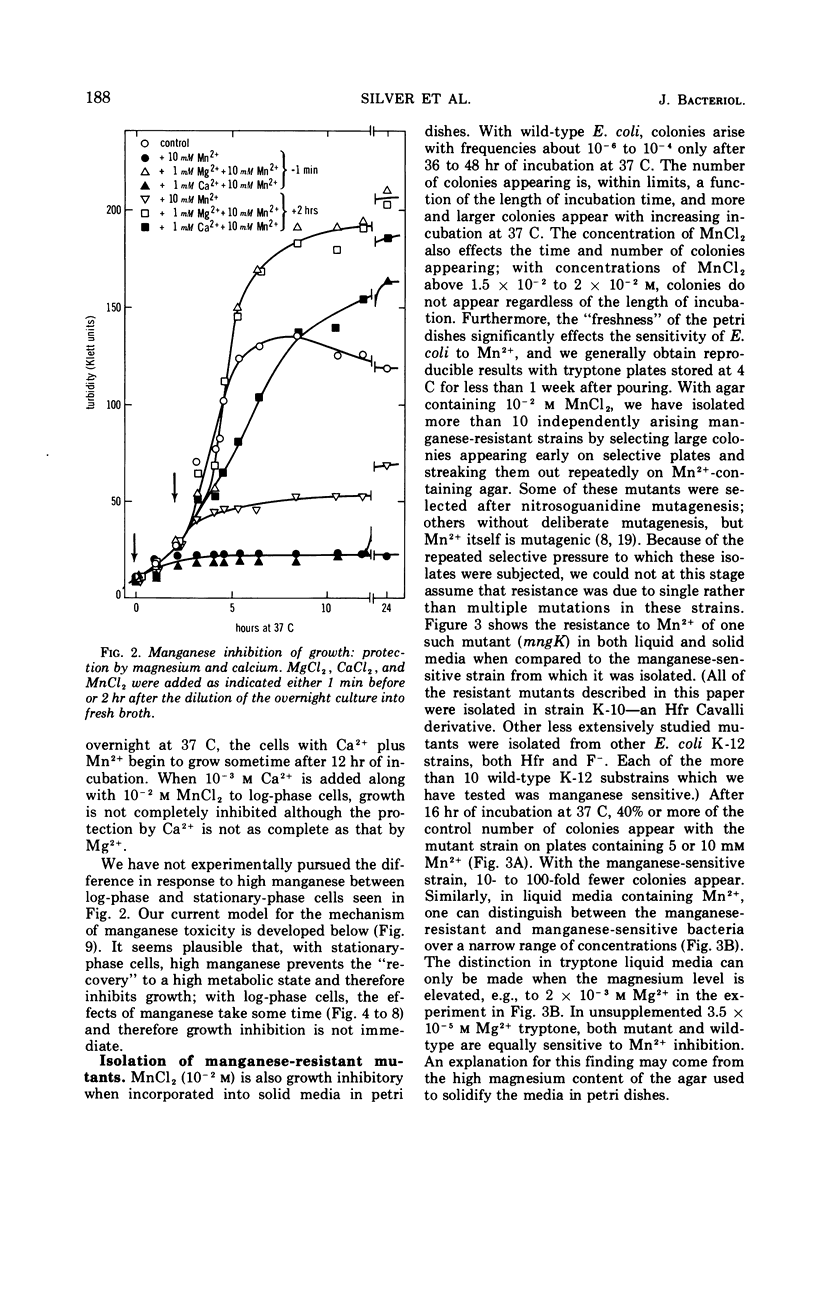
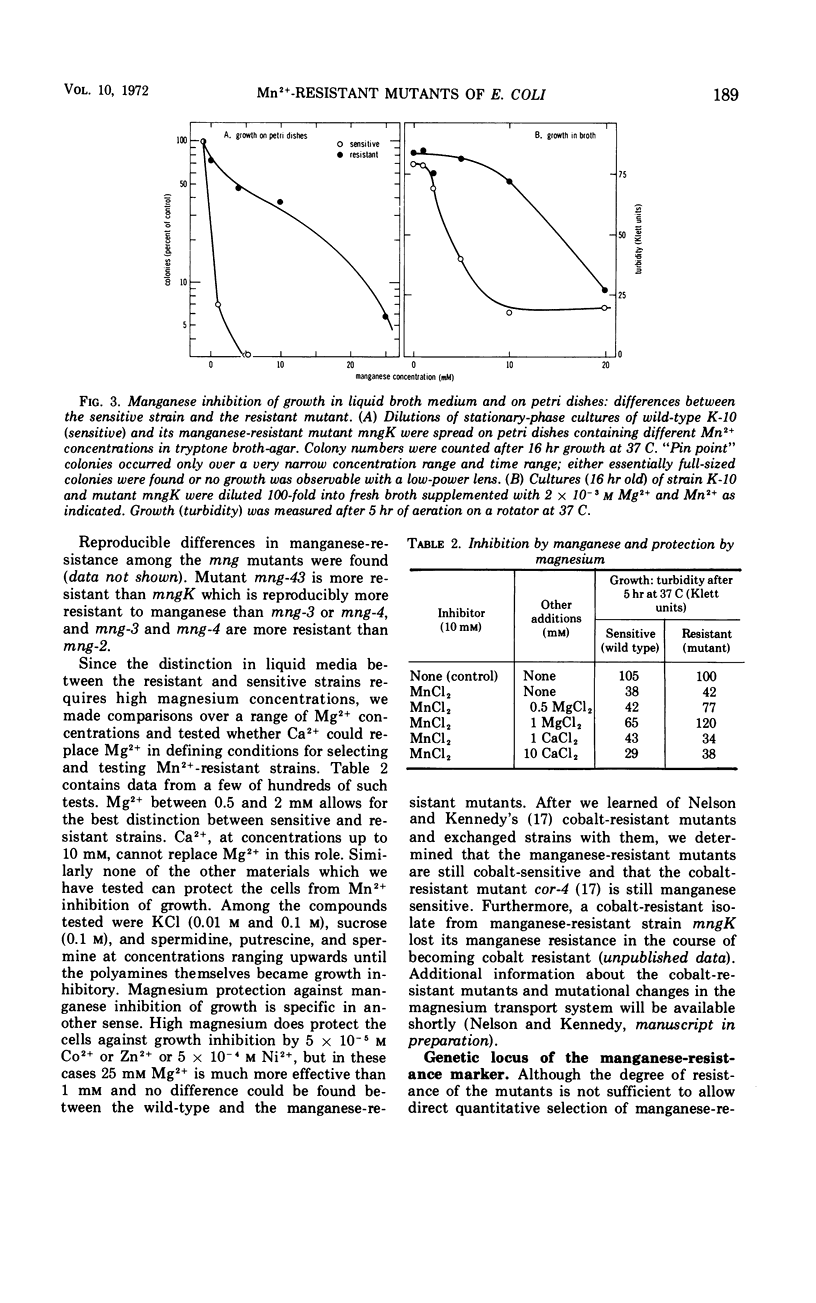
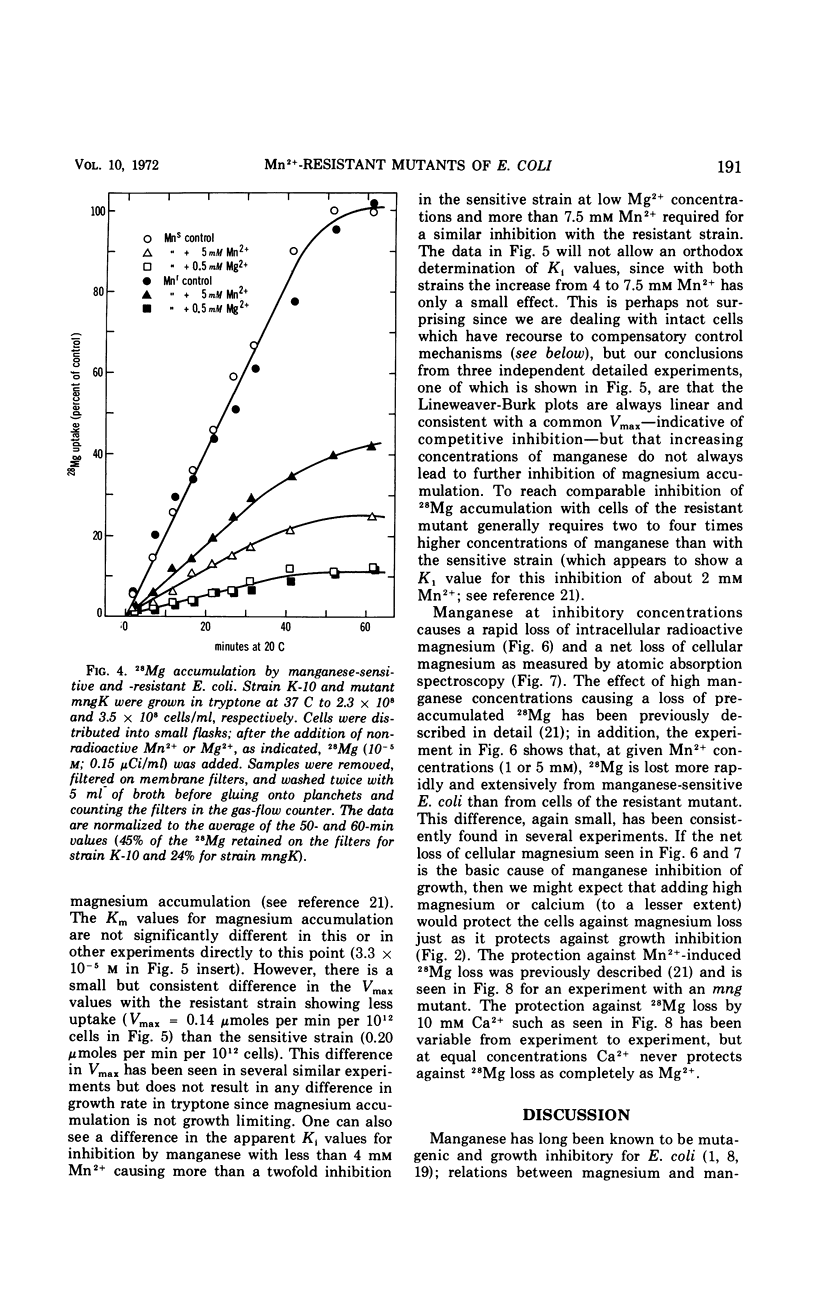
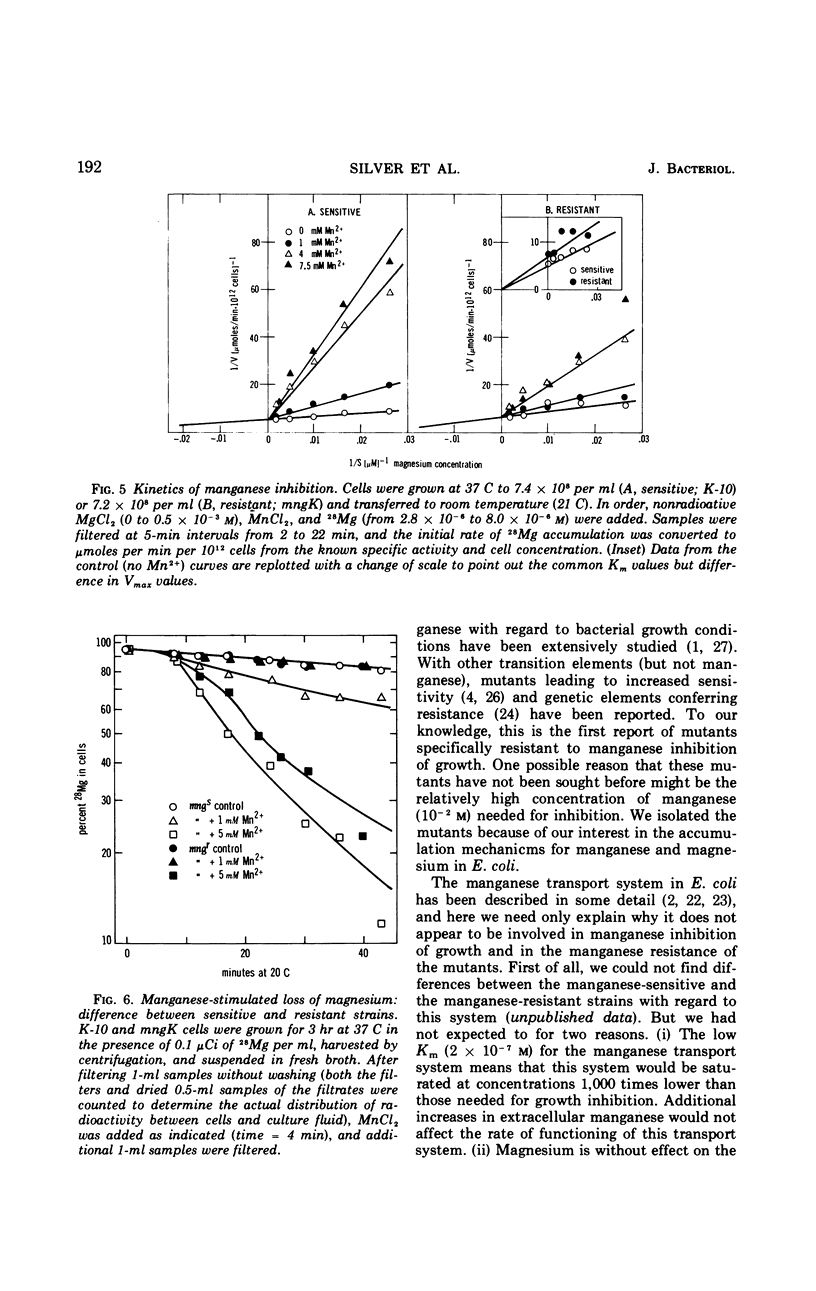
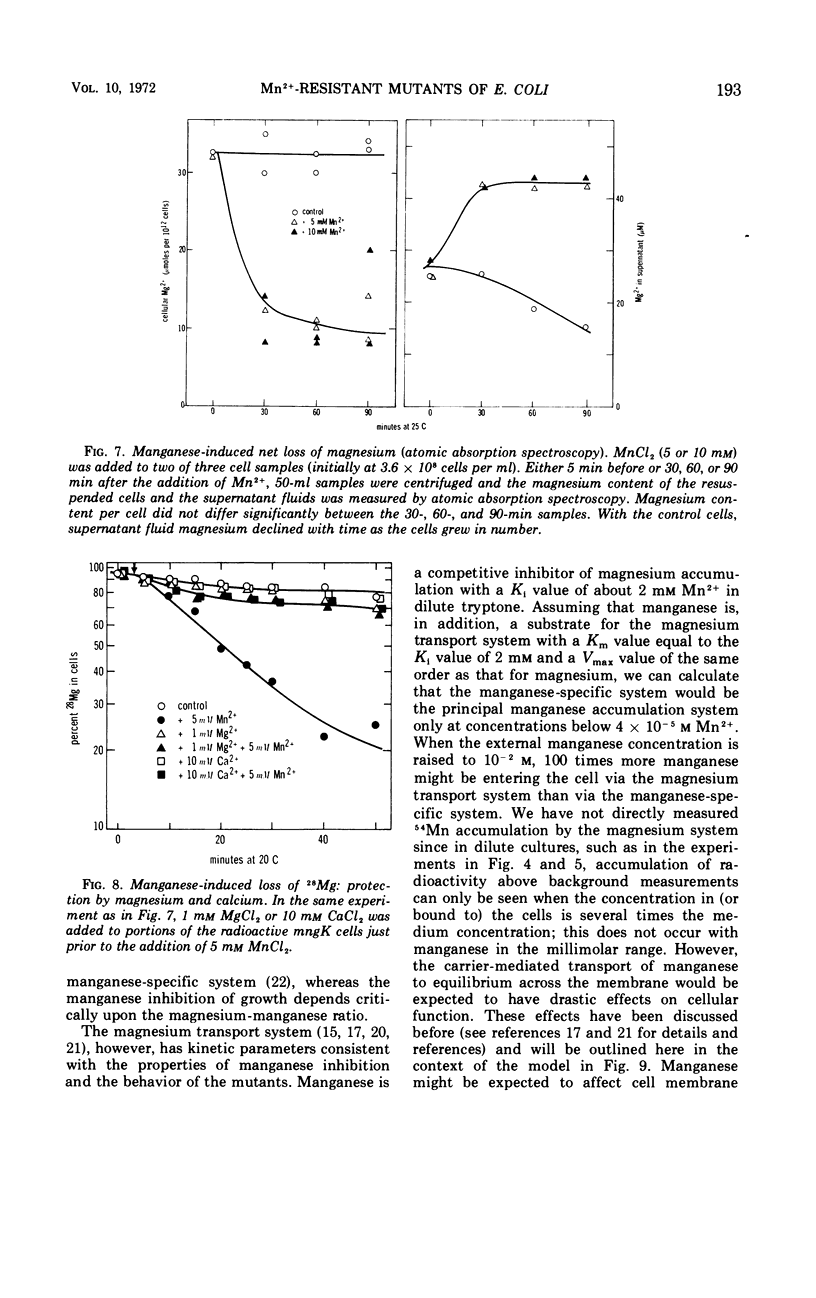
Manganese is growth inhibitory for Escherichia coli. The manganese concentration required for inhibition is dependent upon the magnesium concentration of the medium. Mutants have been isolated which are partially resistant to manganese inhibition in both liquid and solid media. From conjugation experiments, the genetic locus for manganese-resistance, mng, appears to be between 34 and 37 min on the E. coli genetic map. Experiments with radioactive 28Mg lead to the tentative conclusion that the mng mutants are altered in the inhibition constant for manganese as a competitive inhibitor for the mangnesium accumulation system. Once high manganese enters the cells, it displaces internal magnesium and leads to a net cellular loss and hence growth inhibition. The mng mutants are somewhat less subject to manganese-induced magnesium loss under comparable conditions than are manganese-sensitive wild-type cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., ALDOUS E. Ion antagonisms in microorganisms; interference of normal magnesium metabolism by nickel, cobalt, cadmium, zinc, and manganese. J Bacteriol. 1950 Oct;60(4):401–413. doi: 10.1128/jb.60.4.401-413.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. L., Malamy M. H. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970 Jul 27;40(2):496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P. Active Transport of Manganese in Isolated Membranes of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R., Feldman F., Margolin P. Mutation leading to increased sensitivity to chromium in Salmonella typhimurium. J Bacteriol. 1966 Apr;91(4):1509–1515. doi: 10.1128/jb.91.4.1509-1515.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968 Jan;58(1):9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., HANSON J. Mutagenic action of manganese chloride. Cold Spring Harb Symp Quant Biol. 1951;16:215–228. doi: 10.1101/sqb.1951.016.01.017. [DOI] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- Hurwitz C., Rosano C. L. The intracellular concentration of bound and unbound magnesium ions in Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3719–3722. [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Magneisum transport in Escherichia coli. J Biol Chem. 1969 Mar 25;244(6):1653–1655. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971 May 10;246(9):3042–3049. [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R. B., ALDOUS E. Manganese metabolism of Escherichia coli as related to its mutagenic action. Cold Spring Harb Symp Quant Biol. 1951;16:229–231. doi: 10.1101/sqb.1951.016.01.018. [DOI] [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Clark D. Magnesium transport in Escherichia coli. J Biol Chem. 1971 Feb 10;246(3):569–576. [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Kralovic M. L. Manganese accumulation by Escherichia coli: evidence for a specific transport system. Biochem Biophys Res Commun. 1969 Mar 10;34(5):640–645. doi: 10.1016/0006-291x(69)90786-4. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: relationship between chromium sensitivity and high iron requirement in mutants of Escherichia coli. J Bacteriol. 1969 Jun;98(3):1135–1141. doi: 10.1128/jb.98.3.1135-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. The influence of certain trace metals on bacterial growth and magnesium utilization. J Gen Microbiol. 1968 May;51(3):325–335. doi: 10.1099/00221287-51-3-325. [DOI] [PubMed] [Google Scholar]
- Webb M. The mechanism of acquired resistance to Co2+ and Ni2+ in Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):440–446. doi: 10.1016/0304-4165(70)90134-0. [DOI] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


