Abstract
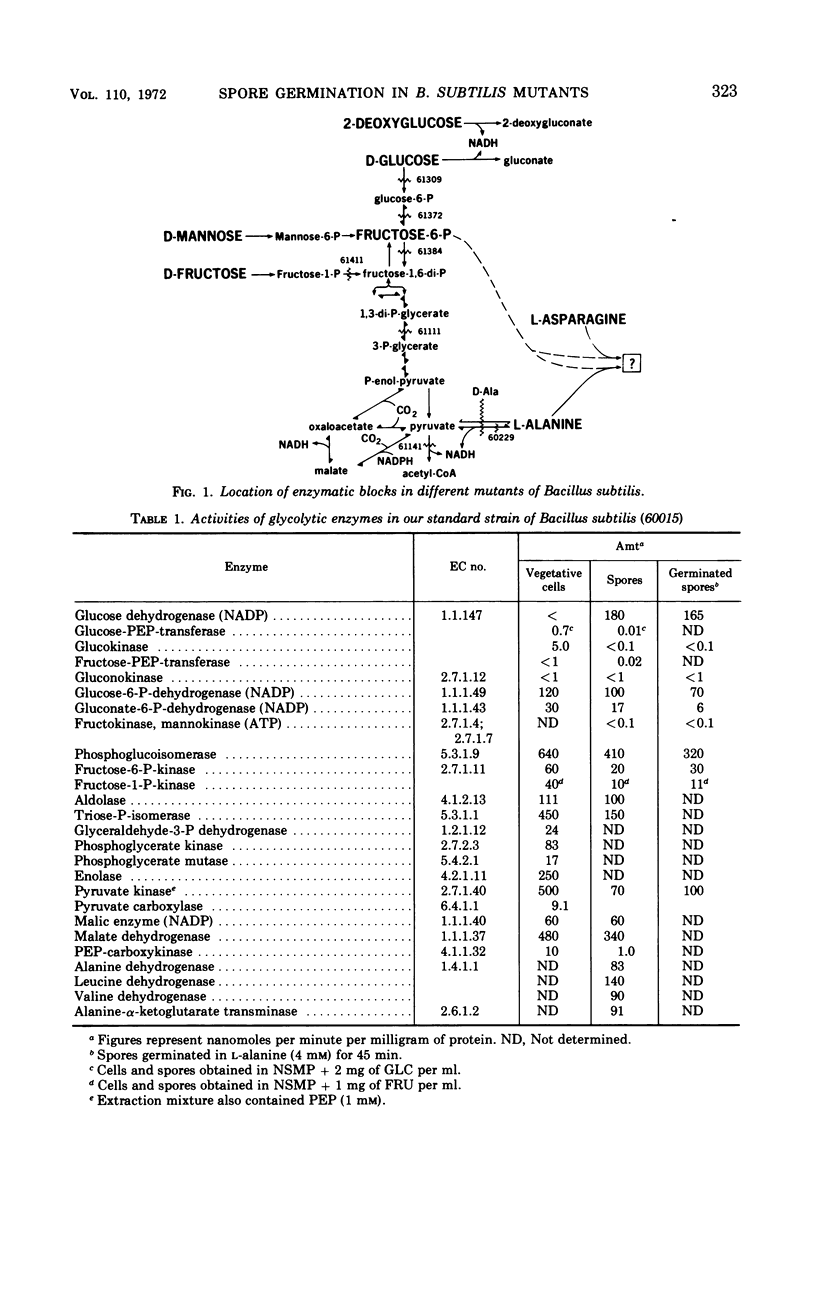
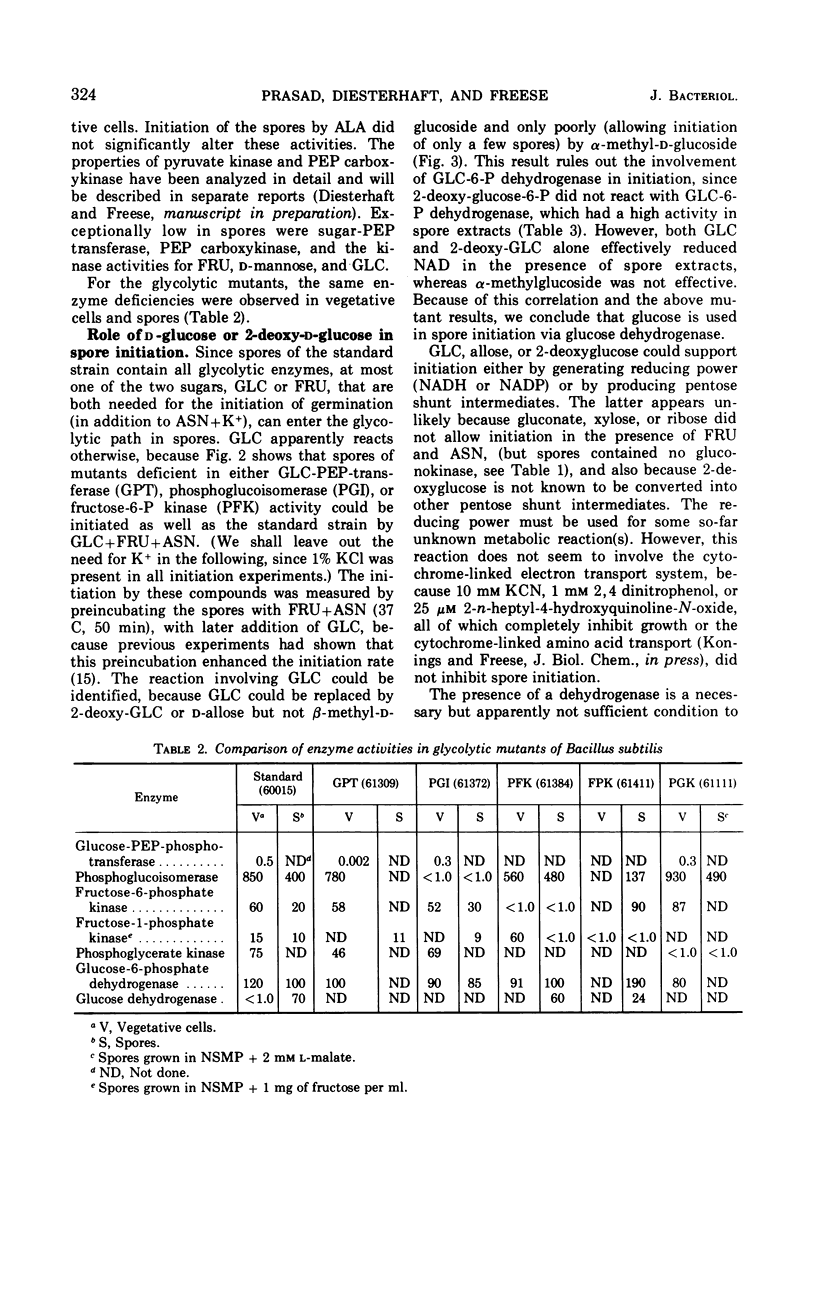
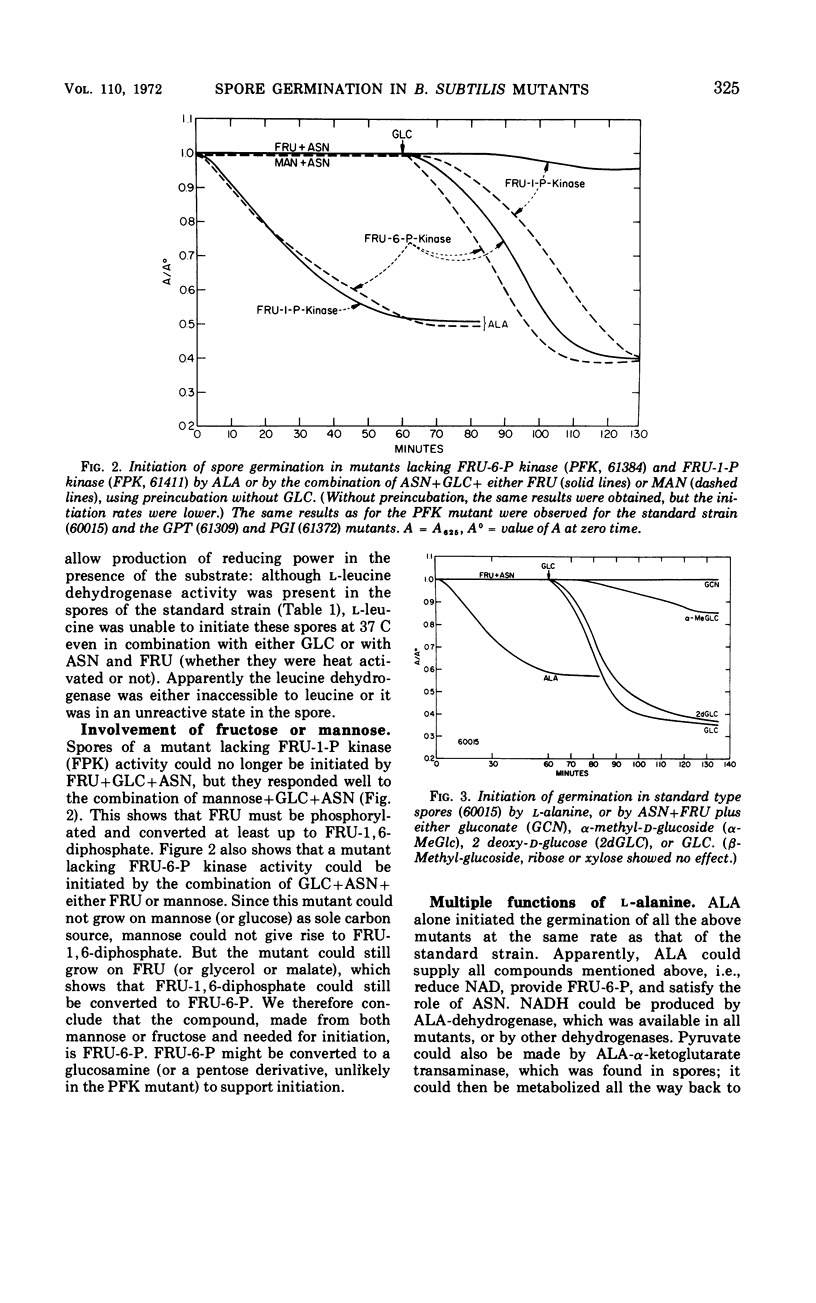
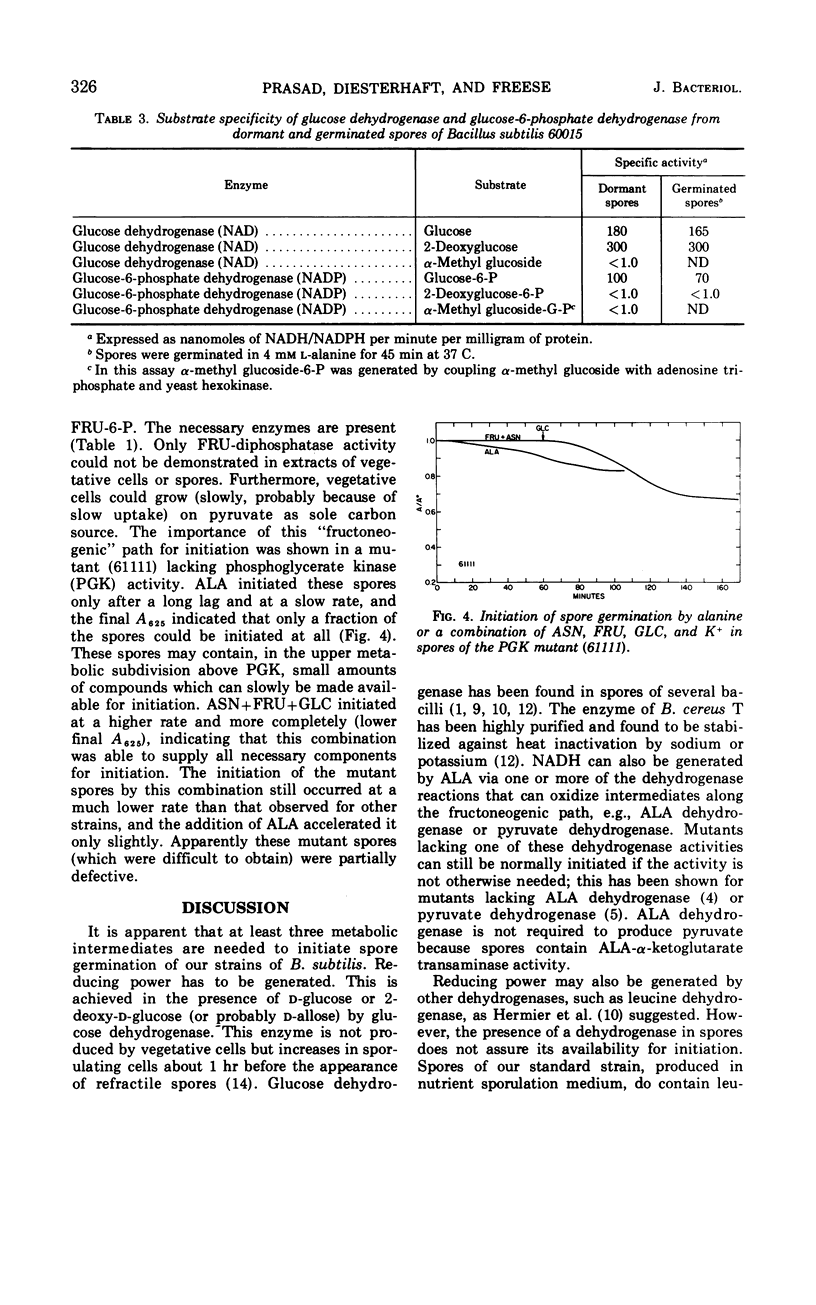
Enzyme activities of glycolysis and glyconeogenesis are present in spores of Bacillus subtilis, the rate-limiting step of glucose (GLC) metabolism being its phosphorylation. GLC allows initiation of germination in the presence of fructose (FRU) and asparagine (ASN), not because it is used via the Embden-Meyerhof path, but because it is oxidized in the nonphosphorylated form via the spore-specific GLC dehydrogenase. Spores of mutants lacking GLC-phosphoenolpyruvate transferase, FRU-6-P-kinase, or phosphoglucoisomerase activity can still be initiated by the above substrate combination. Furthermore, GLC can be replaced by 2-deoxy-GLC, which is also oxidized by GLC-dehydrogenase, but not by α- or β-methylglucoside, which are not substrates of this enzyme. GLC probably acts by reducing nicotinamide adenine dinucleotide (or nicotinamide adenine dinucleotide phosphate), which is used for some metabolic reaction other than the cytochrome-linked electron transport system, since inhibitors of this system do not inhibit initiation. Spores of a mutant lacking FRU-1-P-kinase activity can no longer be initiated by GLC+FRU+ASN, but they do respond to the combination of GLC+mannose+ASN. Since spores of a FRU-6-P-kinase (or phosphoglucoisomerase) mutant can still respond to either FRU or mannose, FRU-6-P (or some derivative) apparently is needed for initiation (in addition to reduced nicotinamide adenine dinucleotide and an amino donor). Alanine can initiate germination in spores of all of the above mutants, indicating that it can form all required compounds. However, in a mutant lacking P-glycerate kinase activity, alanine initiates only after a long lag and at a slow rate, indicating that some compound in the upper metabolic subdivision is required for initiation, in agreement with the above findings. All initiating agents of B. subtilis probably produce the same required compound(s) by different metabolic routes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., PARK S. W., CASHEL M. THE DEVELOPMENTAL SIGNIFICANCE OF ALANINE DEHYDROGENASE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1164–1172. doi: 10.1073/pnas.51.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster H. F., Foster J. W. Response of Bacillus spores to combinations of germinative compounds. J Bacteriol. 1966 Mar;91(3):1168–1177. doi: 10.1128/jb.91.3.1168-1177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969 Sep;99(3):745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- HACHISUKA Y., SUGAI K. Studies on spore germination. IV. Relationship between germination and appearance of glucose dehydrogenase activity in B. subtilis spore. Jpn J Microbiol. 1959 Apr;3:211–222. doi: 10.1111/j.1348-0421.1959.tb00117.x. [DOI] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. EFFECT OF SUGARS AND OTHER CARBON COMPOUNDS ON GERMINATION AND POSTGERMINATIVE DEVELOPMENT OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Nov;88:1403–1415. doi: 10.1128/jb.88.5.1403-1415.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermier J., Rousseau M., Zévaco C. Role des déshydrogénases à nicotinamide adénine dinucléotide dans la phase initiale de la germination chez la spore de Bacillus subtilis. Ann Inst Pasteur (Paris) 1970 May;118(5):611–625. [PubMed] [Google Scholar]
- Sundaram T. K., Cazzulo J. J., Kornberg H. L. Anaplerotic CO2 fixation in mesophilic and thermophilic bacilli. Biochim Biophys Acta. 1969 Nov 18;192(2):355–357. doi: 10.1016/0304-4165(69)90377-8. [DOI] [PubMed] [Google Scholar]
- WOESE C. R. Effect of withholding glutamic acid and asparagine on the germination of spores of Bacillus subtilis. J Bacteriol. 1959 Jun;77(6):690–694. doi: 10.1128/jb.77.6.690-694.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E., Cashel M. Separation of two functional roles of L-alanine in the initiation of Bacillus subtilis spore germination. J Bacteriol. 1967 Sep;94(3):522–529. doi: 10.1128/jb.94.3.522-529.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of L-alanine. J Bacteriol. 1968 Feb;95(2):433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A. PURIFICATION AND CHEMICAL CHARACTERIZATION OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1113–1117. [PubMed] [Google Scholar]


