Abstract
Antagonists of growth hormone-releasing hormone (GHRH) inhibit the growth of various cancers in vivo. This effect is thought to be exerted through suppression of the pituitary growth hormone–hepatic insulin-like growth factor I (IGF-I) axis and direct inhibition of autocrine/paracrine production of IGF-I and -II in tumors. However, other evidence points to a direct effect of GHRH antagonists on tumor growth that may not implicate IGFs, although an involvement of GHRH in the proliferation of cancer cells has not yet been established. In the present study we investigated whether GHRH can function as an autocrine/paracrine growth factor in small cell lung carcinoma (SCLC). H-69 and H-510A SCLC lines cultured in vitro express mRNA for GHRH, which apparently is translated into peptide GHRH and then secreted by the cells, as shown by the detection of GHRH-like immunoreactivity in conditioned media from the cells cultured in vitro. In addition, the levels of GHRH-like immunoreactivity in serum from nude mice bearing H-69 xenografts were higher than in tumor-free mice. GHRH(1–29)NH2 stimulated the proliferation of H-69 and H-510A SCLCs in vitro, and GHRH antagonist JV-1–36 inhibited it. JV-1–36 administered s.c. into nude mice bearing xenografts of H-69 SCLC reduced significantly (P < 0.05) tumor volume and weight, after 31 days of therapy, as compared with controls. Collectively, our results suggest that GHRH can function as an autocrine growth factor in SCLCs. Treatment with antagonistic analogs of GHRH may offer a new approach to the treatment of SCLC and other cancers.
Keywords: cancer therapy, insulin-like growth factor, insulin-like growth factor receptor, lung tumors
Lung cancer is the leading cause of cancer-related deaths in the Western world. Small cell lung carcinoma (SCLC) is a subset of lung cancer that accounts for about 20% of cases and is characterized by poor prognosis resulting from the limited therapeutic options available when the disease is diagnosed (1).
Antagonistic analogs of growth hormone-releasing hormone (GHRH) inhibit the growth of various cancers, such as osteosarcomas (2), glioblastomas (3), SCLC and non-SCLC (4), prostatic (5, 6), renal (7), pancreatic, colorectal (8, 9), and breast cancers (10). This effect is exerted in part by endocrine mechanisms through the inhibition of growth hormone (GH) release from the pituitary, which, in turn, results in the reduction of the hepatic production of insulin-like growth factor I (IGF-I) (11). IGF-I is a potent mitogen for various cancers, including SCLC (12). Other evidence, based on the significant reduction in concentrations of IGF-I and/or IGF-II in osteosarcomas (2) and non-SCLC (4), renal (7), prostatic (5, 6), pancreatic, and colorectal cancers (8, 9) and a decrease in expression of mRNA for IGF-II in tumors after treatment of nude mice with GHRH antagonists, suggests that the inhibition of tumor growth may be the result of direct effects of GHRH antagonists on autocrine/paracrine production of IGFs in tumors (2, 5, 6, 11). This view is supported by the observation that antagonists of GHRH inhibit the proliferation of various human cancer cell lines cultured in vitro, suppress the production of IGF-II, and decrease the telomerase activity (13, 14). GHRH antagonists also could inhibit tumor growth directly by blocking the action of tumoral GHRH by mechanisms independent of IGFs.
Although the expression of GHRH in primary tumors and increased GHRH-like immunoreactivity in the serum of lung cancer patients have been demonstrated (15–17), the function of GHRH in tumorigenesis remains obscure. The aim of our study was to investigate the role of GHRH in the growth of H-69 and H-510A human SCLC. First, we investigated whether H-69 and H-510A cells express and secrete GHRH. Then, we evaluated the effects of GHRH(1–29)NH2 and potent antagonistic analog of GHRH JV-1–36 (18) on cell proliferation in vitro. We also studied the antitumor activity of GHRH antagonist JV-1–36 in nude mice bearing xenografted H-69 cells. The effects of the treatment with GHRH antagonists on serum levels of GHRH, IGF-I, and IGF-II also were evaluated.
Materials and Methods
Peptides.
GHRH(1–29)NH2 and GHRH antagonist JV-1–36 ([PhAc-Tyr1,d-Arg2,Phe(4-Cl)6,Arg9,Abu15,Nle27,d-Arg28,Har29]hGH-RH(1–29)NH2) (where PhAc is phenylacetyl, Abu is α-aminobutyric acid, Nle is norleucine, and Har is homoarginine) were synthesized by solid-phase methods and purified as described (18). For daily injections, peptides were dissolved in 0.1% DMSO in sterile 10% propylene-glycol/water solution.
Cell Culture and Cell Proliferation Assay.
The human SCLC cell line NCI-H-69 was obtained from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium (GIBCO) supplemented with 10% FBS, glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 mg/ml), and amphotericin B (100 units/ml) at 37°C in a humidified 95% air/5% carbon dioxide atmosphere. The human SCLC cell line H-510A was a gift from H. Oie (National Cancer Institute–Navy Oncology Unit, Bethesda, MD). Except for the substitution of 10% newborn calf serum for 10% FBS, this cell line was cultured as described above. Cells were passaged weekly and monitored routinely for mycoplasma contamination by using a detection kit (Boehringer Mannheim). All culture media components were purchased from GIBCO. The effect of the peptide analogs on cell proliferation was examined by the MTT [3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay as described (19). Results were calculated as percent T/C, where T = optical density (OD540) of treated cultures [HITES medium (4) plus GHRH analogs] and C = OD540 of untreated cultures (HITES medium alone).
Animals.
Male athymic (NCrnu/nu) nude mice, approximately 6 weeks old on arrival, were obtained from the National Cancer Institute (Bethesda, MD) and housed in laminar airflow cabinets under pathogen-free conditions with a 12-h light/12-h dark schedule and fed autoclaved standard chow and water ad libitum. Their care was in accord with institutional guidelines.
Tumor Growth Study.
Xenografts of H-69 cells were initiated by s.c. injection of 1 × 107 cells into the right flanks of two male nude mice. Tumors resulting after 2 weeks were aseptically dissected and mechanically minced, and 3-mm3 pieces were transplanted s.c. by trocar needle into 5 male animals; tumors resulting after 3 weeks were retransplanted as mentioned above into 20 male animals. Three weeks after the second transplantation, when tumors had grown to a volume of approximately 70 mm3, mice were divided into three experimental groups of four to five animals each and received the following treatment as s.c. injections: group 1 (control), 0.1% DMSO in saline, s.c. (vehicle solution); group 2, GHRH antagonist JV-1–36 at a dose of 10 μg/day per animal; and group 3, GHRH antagonist JV-1–36 at a dose of 20 μg/day per animal. The treatment was continued for 31 days. Tumors were measured once or twice a week with microcalipers, and the tumor volume was calculated as length × width × height × 0.5236 (20). At the end of the experiment, mice were anesthetized with methoxyflurane (Metofane; Pitman–Moore, Mundelein, IL) and sacrificed by decapitation, and trunk blood was collected. The serum was separated and analyzed by RIA. Body weights were recorded and various organs were removed and weighed. Tumors were dissected, cleaned, and weighed.
RIAs for GHRH, IGF-I, IGF-II, and GH.
GHRH was measured by using 125I-GHRH(1–40) (Bachem) as labeled hormone and anti-GHRH(1–40)(SV-95) antibody (Ab) generated in our laboratory at the final dilution of 1:117,000, which cross-reacts 100% with GHRH(1–29)NH2 as described earlier (21). For determination of GHRH in serum and medium, GHRH(1–29)NH2 was used as a standard. The range was 0.025–50 ng per tube.
Serum samples for IGF-I and IGF-II determination were extracted by using a modified acid/ethanol cryoprecipitation method (22) to eliminate most of the IGF-binding proteins that can interfere in the RIA. IGF-I (88-G4, from Genentech) was used as a standard in the range of 2–500 pg per tube and also for iodination by the chloramine-T method. Antibody UB2–495 (a gift from L. E. Underwood and J. van Wyk) obtained from National Institute of Diabetes and Digestive and Kidney Diseases was used at the final dilution of 1:14,000 in the RIA. IGF-II was measured by using recombinant human IGF-II standard (Bachem) in the range of 2–500 pg per tube. IGF-II was iodinated by the lactoperoxidase method and purified by reverse-phase HPLC by using Vydac C18 column. For the assay, Amano mAb (10 mg/ml) against rat IGF-II (23) (Amano International Enzyme, Troy, VA) was used at the final dilution of 1:14,285. This antibody cross-reacts 100% with human IGF-II and rat IGF-II and 10% with human IGF-I (23). mGH was determined by using materials provided by A. F. Parlow (Pituitary Hormones and Antisera Center, Torrance, CA; mouse GH reference preparation AFP10783B, mouse GH antigen AFP10783B, and anti-rat GH-RIA-5/AFP-411S).
Reverse Transcription–PCR for GHRH.
Total RNA was extracted from H-69 tumors or cells by using RNAzolB (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. RNA was reverse-transcribed into cDNA as described previously (14). The PCR amplification of the cDNAs for GHRH and human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was performed as follows. One microliter of the cDNA was amplified in a 50-μl solution containing 10 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 1.7 mM MgCl2, 200 μM of each dNTP, 2.5 units of Taq DNA polymerase, and 0.4 mM each primer. The primers used were 5′-TCCTCTGACTTCAACAGCGACACC-3′ and 5′-TCTCTCTTCCTCTTGTGCTCTTGG-3′ for hGAPDH (14) and 5′-ATTTGAGCAGTGCCTCGGAG-3′ and 5′-TTTGTTCTGCCCACATGCTG-3′ for GHRH (15). PCR consisted of 1 cycle at 95°C for 3 min, 58°C for 1 min, and 72°C for 1 min and, subsequently, 24 (hGAPDH) or 29 (GHRH) cycles of 95°C for 35 sec, 58°C for 40 sec, and 72°C for 40 sec by using a Perkin–Elmer Cetus model 2400 thermocycler. Aliquots of each PCR product were electrophoresed on a 2% agarose gel and stained with ethidium bromide.
Statistical Analyses.
Data are expressed as mean ± SE. Statistical analyses were performed by using the Student two-tailed t test. All P values are based on two-sided hypothesis testing.
Results
Expression of mRNA for GHRH and Secretion of GHRH by H-69 and H-510A Cells Cultured in Vitro.
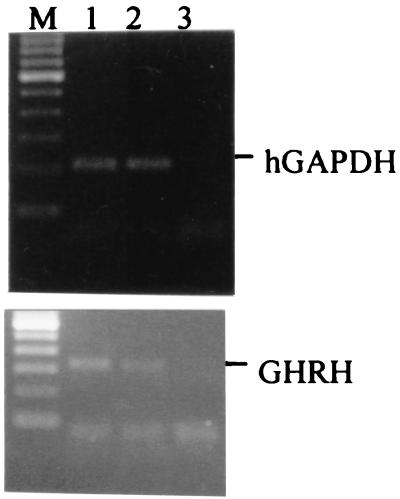
Total RNAs isolated from H-69 and H-510A cells cultured in vitro were subjected to reverse transcription–PCR analysis for the expression of mRNA for GHRH. PCR products were electrophoresed in 2% agarose and stained with ethidium bromide. A 322-bp band, specific for GHRH, was found in both cell lines as illustrated in Fig. 1.
Figure 1.
Expression of mRNA for GHRH and hGAPDH in H-69 (lane 1) and H-510A (lane 2) cells cultured in vitro. Lane 3, negative control. PCRs yielded products of the expected size: 207 bp for hGAPDH and 322 bp for GHRH. M, DNA size marker.
Concentration of the GHRH in samples from culture medium was measured by RIA. Significant amounts of GHRH were detected in medium from H-69 and H-510A cells after 27 h, 72 h, and 168 h of culture as shown in Table 1, whereas culture medium without cells did not contain GHRH at detectable levels. The decrease in GHRH concentration in culture medium from H-510A cells, when the incubation was prolonged to 72–168 h, could be due to inactivation.
Table 1.
Production of GHRH in culture medium from H-69 and H-510A SCLC
| Time, h | GHRH, ng/ml medium
|
|
|---|---|---|
| H-69 | H-510A | |
| 0 | ND | ND |
| 27 | 2.93 | 9.47 |
| 72 | 3.26 | 3.80 |
| 168 | 2.23 | 4.75 |
Aliquots of medium at the indicated periods of time were sujbected to RIA for the detection of GHRH. ND, not detectable.
Effect of GHRH(1–29)NH2 and GHRH Antagonist JV-1–36 on the Proliferation of H-69 and H-510A Cells in Vitro.
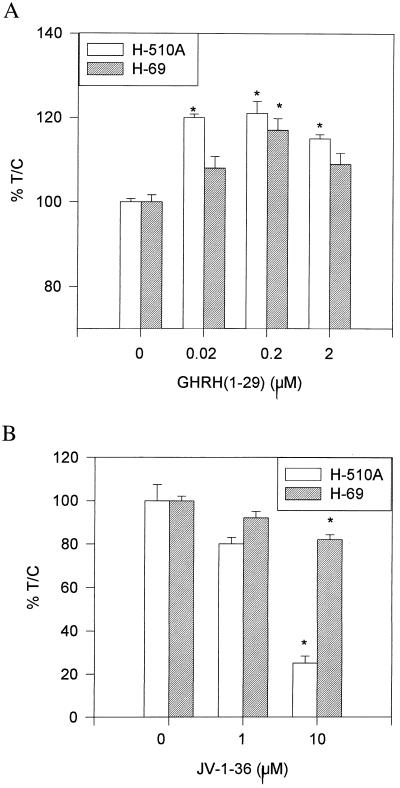
H-69 and H-510A SCLC cells cultured in vitro were exposed to various concentrations of GHRH(1–29)NH2 or GHRH antagonist JV-1–36, and the effect on the proliferation was followed by the MTT assay. As shown in Fig. 2a, GHRH(1–29)NH2 at 2 × 10−7 M stimulated the proliferation of H-69 and H-510A cells by 17% (P < 0.005) and 21% (P < 0.0001), respectively. The growth of H-510A cells also could be stimulated significantly by 2 × 10−8 M and 2 × 10−6 M hormone. H-69 cells showed smaller proliferative responses. GHRH antagonist JV-1–36 at 10−5 M inhibited the proliferation of H-69 and H-510A cells by 18% (P < 0.001) and 75% (P < 0.001), respectively, as compared with controls (Fig. 2b). The inhibition of proliferation was already apparent at 10−6 M JV-1–36.
Figure 2.
Effect of GHRH(1–29) (A) and JV-1–36 on the proliferation of H-510A and H-69 SCLC in vitro. Vertical bars represent SE. *, P < 0.005.
Effect of JV-1–36 on Growth of H-69 SCLC Xenografted into Nude Mice.
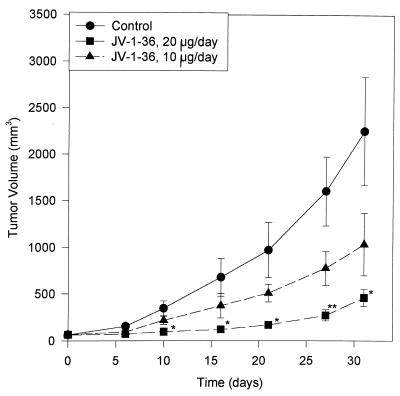
Nude mice bearing xenografts of H-69 SCLC were treated with daily s.c. injections of JV-1–36 at two different dose levels. After 31 days of treatment with JV-1–36 at the dose of 20 μg/day the mean tumor volume was significantly (P < 0.05) reduced to 461 ± 91 mm3, corresponding to a decrease of 80%, as compared with that of the control group (2,254 ± 584 mm3) (Table 2 and Fig. 3). JV-1–36 administered at 10 μg/day per animal also inhibited tumor growth by 54% but this decrease was not significant. The final tumor weights were reduced by 73% (P < 0.05) and 45% (not significant) in the groups treated with JV-1–36 at 20 μg/day and 10 μg/day, respectively, as compared with the control group (Table 2). At the end of the experiment, no significant differences in body weights and the weight of various organs such as lung, heart, liver, and kidneys were observed between the groups, indicating that treatment with JV-1–36 was not toxic for the tumor-bearing animals (data not shown).
Table 2.
Effect of treatment with GHRH antagonist JV-1-36 on tumor volume and weight in nude mice bearing xenografts of H-69 human SCLC
| Treatment | Initial tumor volume, mm3 | Final tumor volume, mm3 | Tumor burden, mg tumor wt/g body wt |
|---|---|---|---|
| Control | 64.8 ± 10.1 | 2,254 ± 584 | 64.7 ± 15.1 |
| JV-1-36 (10 μg/day) | 66.1 ± 15.9 | 1,036 ± 337 | 35.7 ± 12.5 |
| JV-1-36 (20 μg/day) | 65.1 ± 12.7 | 461 ± 91* | 17.7 ± 4.2* |
*P < 0.05.
Figure 3.
Tumor volumes in athymic nude mice bearing s.c. transplanted H-69 SCLC during treatment with GHRH antagonist JV-1–36 administered by daily s.c. injections at doses of 10 μg/day or 20 μg/day per animal. Vertical bars represent SE. *, P < 0.05; **, P < 0.01.
Effect of JV-1–36 on Serum Levels of GHRH in Nude Mice Bearing H-69 SCLC.
RIA for GHRH showed that serum levels of GHRH in nude mice bearing H-69 tumors were about 90% higher than the concentrations in serum of tumor-free animals (Table 3). Treatment of H-69 tumor-bearing animals with GHRH antagonist JV-1–36, at the dose of 20 μg/day per animal, resulted in a 40% (P < 0.05) decrease in serum levels of GHRH compared with the controls receiving vehicle. JV-1–36 administered at 10 μg/day per animal had no effect on the levels of GHRH in the serum.
Table 3.
Serum levels of GHRH in tumor-free nude mice and nude mice bearing xenografts of H-69 SCLC treated with GHRH antagonist JV-1-36 at doses of 20 μg/day or 10 μg/day per animal
| Mice | GHRH, ng/ml |
|---|---|
| Tumor-free | 4.75 ± 0.63 |
| H-69-bearing | |
| Control | 9.2 ± 1.45 |
| JV-1-36 (10 μg/day) | 10.63 ± 1.05 |
| JV-1-36 (20 μg/day) | 5.49 ± 0.61 |
Effect of JV-1–36 on GH, IGF-I, and IGF-II Levels in Serum of Nude Mice Bearing H-69 SCLC.
No significant differences were found in the serum levels of GH, IGF-I, and IGF-II in nude mice bearing H-69 SCLC and treated with GHRH antagonist JV-1–36 at 10 μg/day or 20 μg/day per animal, as compared with the controls.
Discussion
The prognosis for patients with SCLC is poor because of the limited therapeutic options available at the late stages during which the disease is usually diagnosed. The identification of factors that regulate the growth of SCLC would be valuable because it could allow the development of assays for screening patients with SCLC and provide the basis for novel therapeutic approaches.
The autocrine hypothesis for tumor growth postulates that cancer cells can produce hormone-like substances, usually peptides, that are released into the extracellular fluid, act upon the same cells, and stimulate their growth. A classical autocrine agent for SCLC is bombesin (24, 25). The link between bombesin/gastrin-releasing peptide and SCLC was discovered by Cuttitta et al., who found that SCLC cells both secrete and respond to bombesin-like peptides (24). Many SCLC lines also express neuromedin B (25). Our work shows that human SCLC cell lines H-69 and H-510A secrete GHRH and are stimulated by GHRH. This may fulfill the autocrine requirements for GHRH. Both cell lines express GHRH, which stimulates their growth, whereas the exposure to JV-1–36, an antagonistic analog of GHRH, causes growth inhibition in vitro and/or in vivo. Stimulation of growth produced by GHRH(1–29)NH2 was not strikingly high, being approximately 20% for both cell lines exposed to 2 × 10−7 M GHRH(1–29)NH2. However, as these cells produce GHRH, the saturation of the receptors by the endogenous GHRH may obscure the effects of the exogenously added GHRH(1–29)NH2. Thus, JV-1–36, an antagonist of GHRH, produced growth inhibition in vitro in the absence of exogenously added GHRH, likely by blocking the effects of the autocrine GHRH produced by the cells. The quantitation of GHRH secreted by the cells revealed that between 27 h and 168 h of culture, the concentration of GHRH in the culture medium was stable for H-69 or decreased as in the case of H-510A. The decrease in GHRH levels could be due to an enzymatic inactivation.
JV-1–36 is a potent inhibitor of growth of H-69 SCLC tumors in vivo. When nude mice bearing s.c. xenografts of H-69 tumors were treated with JV-1–36 at the dose of 20 μg/day, the tumor volume and weight regressed by 80% and 73%, respectively, after 31 days of treatment compared with controls. The antitumor effect of JV-1–36 on H-69 xenografts in vivo was most likely due to a direct action of the antagonist on the tumor and not exerted through the suppression of the GH/IGF-I axis. The levels of GH, IGF-I, and IGF-II in the serum essentially were unaffected by treatment with JV-1–36. This is probably linked to high levels of GHRH produced by H-69 tumors, which could reduce or nullify the inhibitory effect of JV-1–36 on the pituitary GH and hepatic IGF-I production in tumor-bearing animals. This is in agreement with our finding that GHRH levels in the serum of mice free of tumors were only about 50% of the corresponding levels of GHRH in mice bearing H-69 xenografts. H-510A cells, which showed considerably higher sensitivity to JV-1–36 in vitro than H-69 cells, did not grow in nude mice, and, thus, the antitumor activity of JV-1–36 on this cell line in vivo could not be evaluated.
The receptor that mediates the direct effects of GHRH on cell growth remains to be identified. GHRH receptors, of the pituitary type, are not expressed by H-69 and H-510A cells (unpublished data). GHRH is a member of the family of peptides that includes glucagon, secretin, vasoactive intestinal peptide (VIP), gastric inhibitory peptide, and pituitary adenylate cyclase-activating peptide (PACAP). A considerable structural homology exists between these peptides as well as among the corresponding receptors (26). Thus, GHRH appears to compete for binding to an unknown member of this family of receptors expressed in tumors and one that is likely related to VIP/GHRH receptor proteins.
In conclusion, our results suggest that GHRH is an autocrine growth factor for SCLC lines such as H-69 and H-510A. Further evidence for an IGF-independent mechanism of action of GHRH antagonists on H-69 SCLC, and probably other tumors, is provided by the observation that mRNA levels of IGF-II in H-69 tumors essentially were unaffected by the treatment with JV-1–36, whereas the mRNA for IGF-I was not detectable in H-69 cells (data not shown). Thus, the inhibition of action of endogenous GHRH could be the first step in a complex cascade of events that result in tumor inhibition, which may or may not involve the IGFs (8). The reduction in IGF-I and IGF-II levels observed in many tumors after therapy with GHRH antagonists (8) could be the consequence of an initial blocking of autocrine/paracrine GHRH. The subsequent inhibition of tumor growth then could result from a suppression of IGF-I or IGF-II production. A therapy based on GHRH antagonists could be applied for the treatment of SCLCs and other cancers that appear to depend on the production of GHRH, not only as a means to manipulate the GH/IGF-I axis or suppress tumoral IGF-I and IGF-II, but also to inhibit the autocrine/paracrine stimulation by GHRH. To establish whether this autocrine stimulation is a general feature of primary SCLCs and other cancers, the extension of these investigations to additional cell lines and the screening of primary tumors for the production of biologically active GHRH are required. In addition, epidemiological studies on the levels of circulating GHRH could reveal whether elevated levels of GHRH are associated with an increased risk for lung cancer and other cancers.
Acknowledgments
We thank E. Glotser and H. Valerio for technical assistance. The gifts of materials used in the RIA from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases are appreciated. The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department and a grant from ASTA Medica (Frankfurt on Main, Germany) (all to A.V.S.). Tulane University has applied for patents on GH-RH antagonists cited in this paper, and J.L.V. and A.V.S. are co-inventors on that patent.
Abbreviations
- GH
growth hormone
- GHRH
GH-releasing hormone
- SCLC
small cell lung carcinoma
- IGF-I and IGF-II
insulin-like growth factor I and II, respectively
- hGAPDH
human glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Ihde D C. Chest. 1995;107:243–248. doi: 10.1378/chest.107.6_supplement.243s. [DOI] [PubMed] [Google Scholar]
- 2.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 3.Kiaris, H., Schally, A. V. & Varga, J. L. (2000) Neoplasia, in press. [DOI] [PMC free article] [PubMed]
- 4.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 5.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamharzi N, Schally A V, Koppan M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 9.Szepeshazi, K., Schally, A. V., Groot, K., Armatis, P., Hebert, F. & Halmos, G. (1999) Eur J Cancer., in press. [DOI] [PubMed]
- 10.Kahan, Z., Varga, J. L., Schally, A. V., Rekasi, Z., Armatis, P., Chatzistamou, I., Czompoly, T. & Halmos, G. (2000) Breast Cancer Res. Treat., in press. [DOI] [PubMed]
- 11.Schally A V, Kovacs M, Toth K, Comaru-Schally A M. In: Growth Hormone Secretagogues in Clinical Practice. Bercu B B, Walker R F, editors. New York: Dekker; 1998. pp. 145–162. [Google Scholar]
- 12.Macaulay V M, Everard M J, Teale J D, Trott P A, Van Wyk J J, Smith I E, Millar J L. Cancer Res. 1990;50:2511–2517. [PubMed] [Google Scholar]
- 13.Csernus V J, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiaris H, Schally A V. Proc Natl Acad Sci USA. 1999;96:226–231. doi: 10.1073/pnas.96.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahan Z, Arencibia J M, Csernus V J, Groot K, Kineman R D, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1999;84:582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 16.Rivier J, Spiess J, Thorner M, Vale W. Nature (London) 1982;300:276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- 17.Schopohl J, Losa M, Frey C, Wolfram G, Huber R, Permanetter W, von Pawel J, Muller O A, von Werder K. Clin Endocrinol. 1991;34:463–467. doi: 10.1111/j.1365-2265.1991.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 18.Varga J L, Schally A V, Csernus V J, Zarandi M, Halmos G, Groot K, Rekasi Z. Proc Natl Acad Sci USA. 1999;96:692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plumb J A, Milroy R, Kaye S B. Cancer Res. 1989;49:4435–4440. [PubMed] [Google Scholar]
- 20.Redding T W, Schally A V. Proc Natl Acad Sci USA. 1983;80:1078–1082. doi: 10.1073/pnas.80.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groot K, Csernus V J, Pinski J, Zsigo J, Rekasi Z, Zarandi M, Schally A V. Int J Pept Protein Res. 1993;41:162–168. doi: 10.1111/j.1399-3011.1993.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 22.Breier B H, Gallaher B W, Gluckman P D. J Endocrinol. 1991;128:347–357. doi: 10.1677/joe.0.1280347. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Asami O, Hayano T, Sasaki I, Yoshitake Y, Nishikawa K. Endocrinology. 1989;124:870–877. doi: 10.1210/endo-124-2-870. [DOI] [PubMed] [Google Scholar]
- 24.Cutitta F, Carney D N, Mulshine J W, Moody T W, Fedorko J, Fischler A, Minna J D. Nature (London) 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 25.Spindel E R, Giladi E, Segerson T P, Nagalla S. Recent Prog Horm Res. 1993;48:365–391. doi: 10.1016/b978-0-12-571148-7.50017-8. [DOI] [PubMed] [Google Scholar]
- 26.Christophe J, Svoboda M, Dehaye J P, Winand J, Vandermeers-Pire M C, Vandermeers A, Cauvin A, Gourlet P, Robberecht P. In: Peptide Hormones as Prohormones: Processing, Biological Activity, Pharmacology. Martinez J, editor. New York: Halsted; 1989. pp. 211–242. [Google Scholar]