Abstract
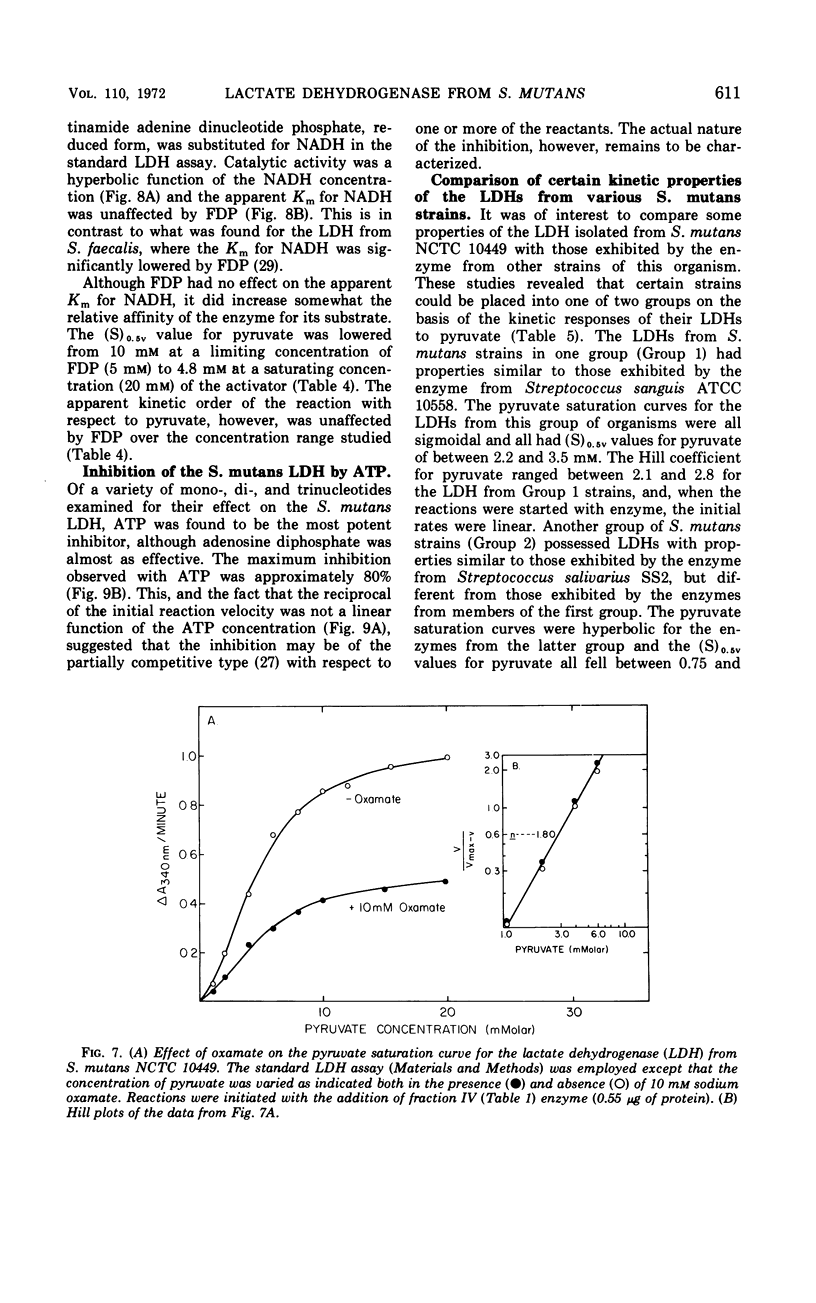
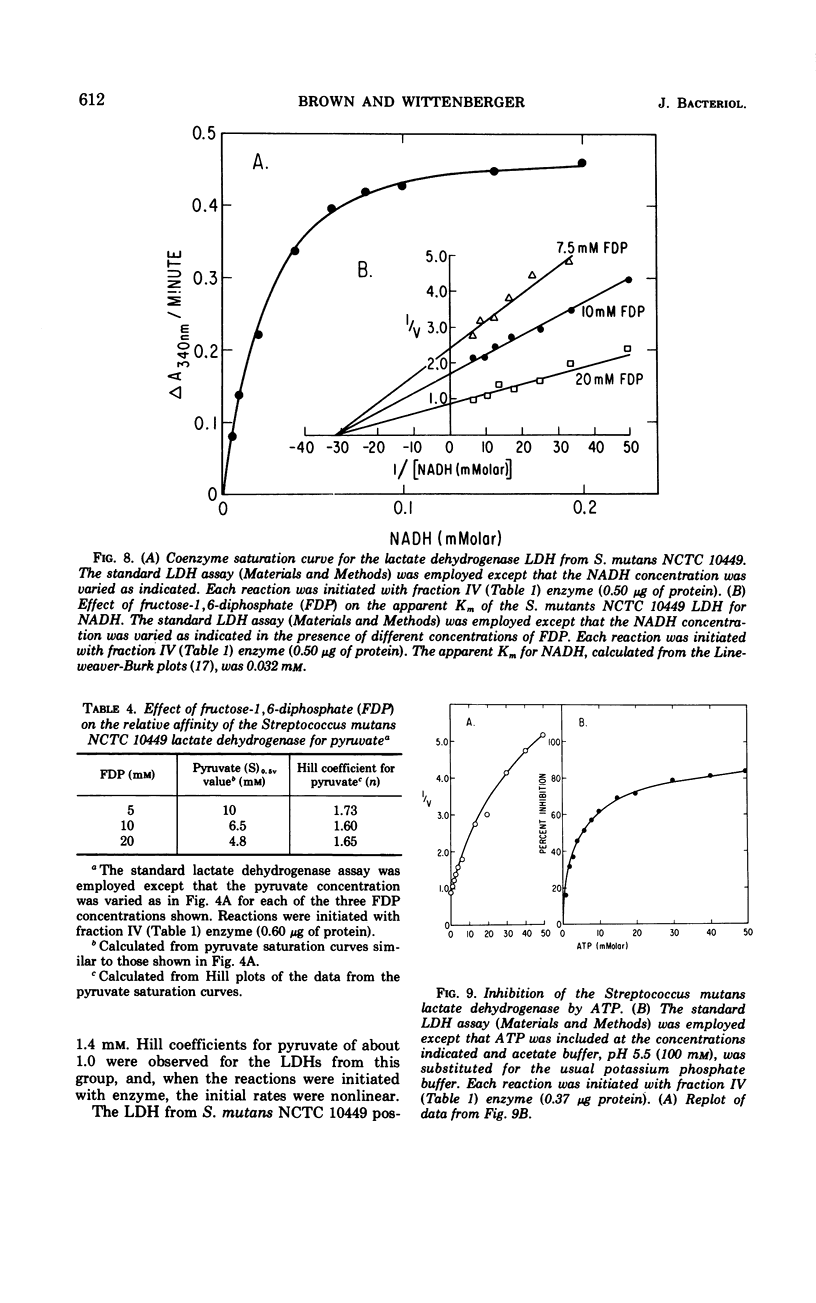
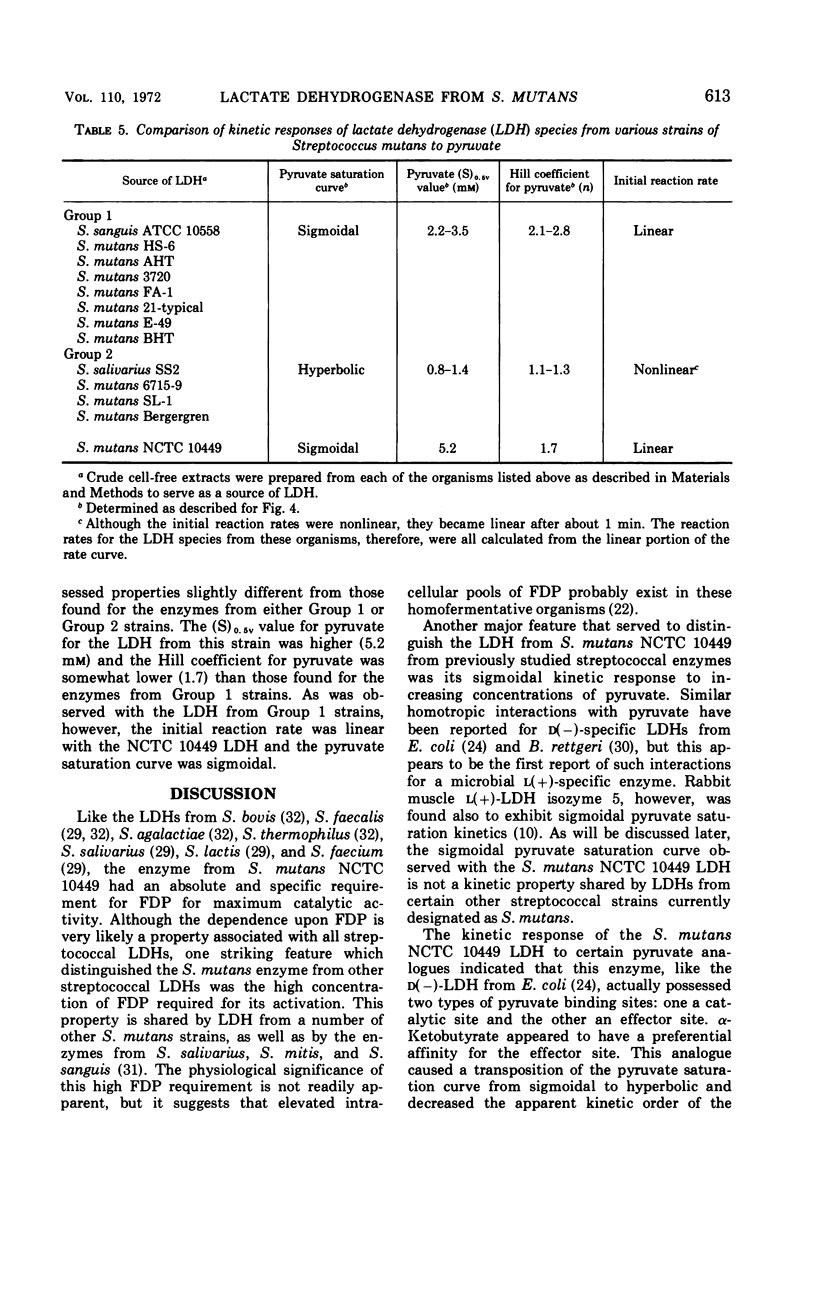
The lactate dehydrogenase (LDH) from Streptococcus mutans NCTC 10449 is under stringent metabolic control. The partially purified enzyme was specifically activated by high concentrations of fructose-1,6-diphosphate (FDP) and was inhibited by adenosine triphosphate. There appeared to be at least two binding sites for the activator which interacted in a cooperative manner. The interaction between the FDP sites was independent of the pH of the assay system, although the relative affinity of the enzyme for the activator was influenced by pH. There also appeared to be at least two pyruvate binding sites on the S. mutans LDH with some cooperative interaction between them, and the interaction between these sites was also independent of the hydrogen ion concentration. Two pyruvate analogues had different effects on the interaction of pyruvate with the LDH. One of the analogues, α-ketobutyrate, stimulated enzyme activity at limiting pyruvate concentrations, but had no significant effect at saturating concentrations of the substrate. The net effect of α-ketobutyrate was to shift the pyruvate saturation curve from sigmoidal to hyperbolic and to decrease the Hill coefficient from about 2.0 to 1.0. The other pyruvate analogue, oxamate, inhibited enzyme activity at all pyruvate concentrations but had no effect on the sigmoidal nature of the pyruvate saturation curve or on the apparent kinetic order of the reaction with respect to substrate. These results suggested that there may be two types of pyruvate binding sites on the LDH from S. mutans. Other kinetic properties of the S. mutans NCTC 10449 enzyme were studied and compared with those exhibited by the LDH from several other strains of the organism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Base composition of deoxyribonucleic acid isolated from cariogenic streptococci. Arch Oral Biol. 1970 Apr;15(4):365–368. doi: 10.1016/0003-9969(70)90063-4. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Genetic heterogeneity in Streptococcus mutans. J Bacteriol. 1971 Apr;106(1):192–196. doi: 10.1128/jb.106.1.192-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNIS D., KAPLAN N. O. D- and L-lactic acid dehydrogenases in Lactobacillus plantarum. J Biol Chem. 1960 Mar;235:810–818. [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Fritz P. J. Rabbit muscle lactate dehydrogenase 5; a regulatory enzyme. Science. 1965 Oct 15;150(3694):364–366. doi: 10.1126/science.150.3694.364. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B. D(-)-lactate dehydrogenases in fungi. Kinetics and allosteric inhibition by guanosine triphosphate. J Biol Chem. 1971 Apr 10;246(7):2116–2126. [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- PAPACONSTANTINOU J., COLOWICK S. P. The role of glycolysis in the growth of tumor cells. I. Effects of oxamic acid on the metabolism of Ehrlich ascites tumor cells in vitro. J Biol Chem. 1961 Feb;236:278–284. [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Interacting binding sites of L-specific lactic dehydrogenase of Escherichia coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):379–383. doi: 10.1016/0006-291x(65)90205-6. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Fulco J. G. Purification and allosteric properties of a nicotinamide adenine dinucleotide-linked D(-)-specific lactate dehydrogenase from Butyribacterium rettgeri. J Biol Chem. 1967 Jun 25;242(12):2917–2924. [PubMed] [Google Scholar]
- Wittenberger C. L. Kinetic studies on the inhibition of a (D(-)-specific lactate dehydrogenase by adenosine triphosphate. J Biol Chem. 1968 Jun 10;243(11):3067–3075. [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stouthamer A. H. Fermentation of glucose, lactose, galactose, mannitol, and xylose by bifidobacteria. J Bacteriol. 1968 Aug;96(2):472–478. doi: 10.1128/jb.96.2.472-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


