Abstract
Prion diseases are fatal neurodegenerative conditions for which there is no effective treatment. Prion propagation involves the conversion of cellular prion protein, PrPC, to its conformational isomer, PrPSc, which accumulates in disease. Here, we show effective therapeutic knockdown of PrPC expression using RNAi in mice with established prion disease. A single administration of lentivirus expressing a shRNA targeting PrP into each hippocampus of mice with established prion disease significantly prolonged survival time. Treated animals lived 19% and 24% longer than mice given an “empty” lentivirus, or not treated, respectively. Lentivirally mediated RNAi of PrP also prevented the onset of behavioral deficits associated with early prion disease, reduced spongiform degeneration, and protected against neuronal loss. In contrast, mice receiving empty virus or no treatment developed early cognitive impairment and showed severe spongiosis and neuronal loss. The focal use of RNAi therapeutically in prion disease further supports strategies depleting PrPC, which we previously established to be a valid target for prion-based treatments. This approach can now be used to define the temporal, quantitative, and regional requirements for PrP knockdown for effective treatment of prion disease and to explore mechanisms involved in predegenerative neuronal dysfunction and its rescue.
Keywords: behavior, gene therapy, neurodegeneration
In the absence of endogenous PrPC, PrPSc cannot replicate, and PrP-null mice are resistant to prion infection (1–3). Previously, we established that PrPC is a valid therapeutic target in prion disease. We showed that depleting endogenous neuronal PrPC, through Cre-mediated recombination at the genomic level, reversed early spongiform pathology in prion-infected mice and in the long term prevented both neuronal loss and development of clinical disease (4). Further, we found that early pathology was associated with neuronal functional impairment before irreversible neuronal or even synaptic loss occurred, and that this was rescued by PrP depletion (5). Thus, early pathology was associated with early symptoms, which could be cured. However, PrP knockdown in this model did not offer direct therapeutic possibilities because of the transgene-mediated mechanism of PrPC depletion (6). Potential treatments aimed at achieving the same effect require either ligand or drug-mediated means of preventing PrPC conversion or somatic gene manipulation, such as reduction of PrP expression through gene silencing. RNAi has emerged as an increasingly powerful tool for therapeutic gene silencing and viral vectors expressing shRNAs have been successfully used as gene therapy in several animal models of neurodegenerative disorders (7–10). Lentiviruses, in particular, are able to transduce postmitotic cells and give rise to long-term expression of shRNAs in neurons when delivered intracerebrally.
Therapeutic use of this technology in prion disease has not been reported, although knockdown of PrP expression by RNAi in vitro (11–13) and in vivo (13) has been described.
Here, we show that stereotaxic hippocampal injection of lentiviruses expressing anti-PrP shRNAs in mice with established prion disease rescues neuronal function, protects against pathological and behavioral disease progression, and prolongs survival.
Results
Lentivirally Mediated RNAi Reduces PrP Expression in Vitro and in Vivo.
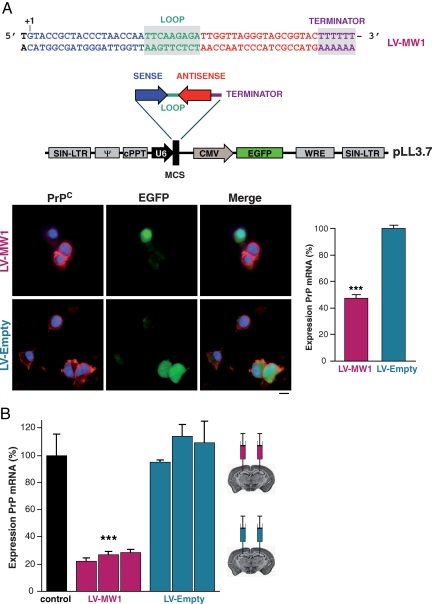
siRNA sequences targeting PrP were screened in vitro for efficacy of knockdown (data not shown). The most effective sequence, MW1, markedly reduced PrP mRNA and protein expression in N2a and GT-1 neuronal cell lines and prevented accumulation of PrPSc in prion-infected cells [supporting information (SI) Fig. S1]. An shRNA corresponding to the MW1 sequence was cloned into the lentiviral vector pLL3.7, which also expresses EGFP reporter protein (14) to generate the anti-PrP lentivirus, LV-MW1 (Fig. 1A). A control virus, LV-Empty, with no shRNA insert was also generated. The lentiviruses were pseudotyped with the VSV-G coat protein to increase neuronal tropism and produced as described (15). Transduction of neuronal cell lines confirmed the LV-MW1 virus reduced PrP protein and mRNA (Fig. 1A, P = 0.0014; Student's t test, two tails), whereas the control virus LV-Empty did not.
Fig. 1.
Lentiviruses expressing anti-PrP shRNAs reduce PrP protein and mRNA levels in vitro and in vivo. (A) Oligonucleotide insert encoding shRNA targeting PrP was cloned into the pLL3.7 lentivector, which also expresses EGFP reporter protein to generate virus LV-MW1. A control lentivirus LV-Empty lacking the shRNA insert was also produced. Transduction of N2a cells with LV-MW1 markedly reduced PrPC expression detected with anti-PrP antibody ICSM18 (red) in transduced cells (green), but LV-Empty had no effect. Cell nuclei were stained with DAPI (blue). RT-PCR showed LV-RNAi-mediated reduction of PrP mRNA by LV-MW1 but not LV-Empty (P = 0.0014; Student's t test, two tails). Error bars represent SEM. Three replicates were performed for each sample. All data shown are 4 days after transduction. (B) PrP mRNA levels were reduced in the hippocampi of uninfected FVB mice 2 weeks after treatment with LV-MW1 (P < 0.0001; Student's t test, two tails) but not with LV-Empty (n = 3 in each case) compared with total levels in uninjected control mice. Error bars and replicates as above.
In vivo, bilateral hippocampal injections of LV-MW1 in wild-type uninfected mice reduced PrP mRNA by ≈80% of normal values 2 weeks after treatment in whole hippocampi (P < 0.0001; Student's t test, two tails), whereas LV-Empty was ineffective (Fig. 1B).
Early Behavioral Symptoms in Prion-Infected Mice Are Rescued After Lentivirally Mediated Knockdown of Hippocampal PrP Expression.
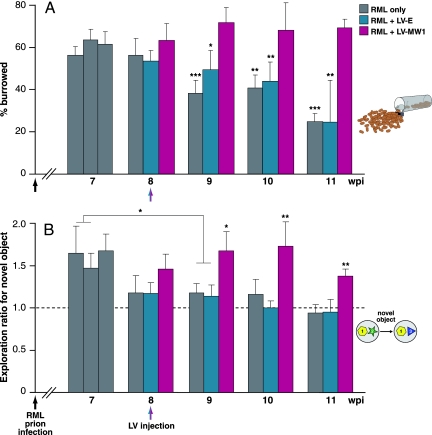
We then tested the lentiviruses in prion-infected mice. We used transgenic tg37 mice (6) for these experiments, because they allowed us to directly compare therapeutic efficacy of lentiviral-mediated RNAi with the effects of adult onset Cre-mediated PrPC depletion in prion infection. Early spongiosis is associated with impairment of hippocampal-dependent behavioral tasks, specifically “burrowing” (16, 17) and object recognition memory, both of which recover after transgene-mediated PrP depletion (5). We infected tg37 mice intracerebrally with RML (Rocky Mountain Laboratories) prions at 1 week of age. Eight weeks later, we stereotaxically injected either LV-MW1 or LV-Empty into both hippocampi. A third group of mice received no lentivirus (n = ≈20 for each group). All three groups were tested in burrowing and object recognition tasks from 7 weeks postinfection (wpi). Lentiviral RNAi of PrP prevented the loss of burrowing activity seen in mice treated with LV-Empty or with no virus (Fig. 2A). All mice burrowed equally at 7 and 8 wpi, but mice treated with LV-Empty or with no virus showed a significant decline in burrowing activity by 9 wpi compared with LV-MW1 treated mice (P = 0.011 for LV-Empty, and P = 0.0001 for no virus). Burrowing activity continued to decline in LV-Empty and untreated mice, but mice given LV-MW1 remained active with sustained levels of burrowing throughout (P < 0.005 for LV-MW1 compared with LV-Empty and untreated mice at all time points after 9 wpi). There was no significant difference in burrowing activity between LV-Empty and untreated mice at 9 wpi (P = 0.098) or at later time points (P = 0.786 at 10 wpi and 0.449 at 11 wpi). (In all cases, statistical analysis was by Student's t test, two tails, unequal variance.)
Fig. 2.
Lentivirally mediated RNAi of PrP prevents loss of burrowing behavior and memory deficits in prion-infected mice. (A) Mice were infected with RML prions at 1 week of age (black arrows) and tested for their ability to actively displace (burrow) food pellets from a tube over 24 h from 7 weeks after inoculation. At 8 wpi, mice were treated with LV-MW1 or LV-Empty or with no virus (red and blue arrows). LV-MW1-treated mice continued to burrow actively, whereas LV-Empty and untreated mice lost burrowing behavior (P = 0.011 and P = 0.0001, respectively, at 9 wpi and P < 0.005 for LV-MW1 compared with LV-Empty-treated and untreated mice at all time points after 9 wpi). (B) Mice were also tested for object recognition memory. LV-MW1-treated mice retained the capacity to distinguish novel objects, whereas this was lost in LV-Empty-treated mice and untreated animals (P = 0.021 and 0.027, respectively, for exploration at 7 wpi compared with 9 wpi in these groups). In contrast, mice treated with LV-MW1 retained object-recognition memory (P = 0.016 and 0.014, compared with LV-Empty-treated and untreated mice, respectively, at 9 wpi), and this was sustained for the course of the experiment (P < 0.005 for all groups at 10 and 11 wpi). Dashed line (exploratory ratio = 1) indicates random exploration of both objects, denoting no memory. n = 22 for LV-MW1 and n = 18 for LV-Empty and RML-only-treated groups.
Lentiviral RNAi of PrP in the hippocampus also protected against loss of object recognition memory (Fig. 2B). Healthy rodents exposed to an object they have not previously seen will explore it more actively than a familiar object (18, 19). Random exploration of two objects results in an exploratory ratio of 1, whereas preferential exploration of the novel object gives a ratio >1. All groups of infected mice explored novel objects preferentially at 7 wpi, but by 8 wpi, object recognition memory was lost in LV-Empty and untreated mice (P = 0.021 and 0.027, respectively, for exploration at 7 compared with 9 wpi). In contrast, mice treated with LV-MW1 retained object recognition memory (P = 0.016 and 0.014, compared with LV-Empty and untreated mice, respectively, at 9 wpi), and this was sustained for the course of the experiment (P < 0.005 for all groups at 10 and 11 wpi). Testing was stopped at 11 wpi, because by 12 wpi, all LV-Empty and untreated mice became clinically sick, and some of the LV-MW1-treated animals showed early scrapie symptoms. (In all cases, statistical analysis was by Student's t test, two tails, unequal variance.)
Lentivirally Mediated Knockdown of PrP Prolongs Survival in Prion-Infected Mice and Is Neuroprotective After a Single Hippocampal Administration.
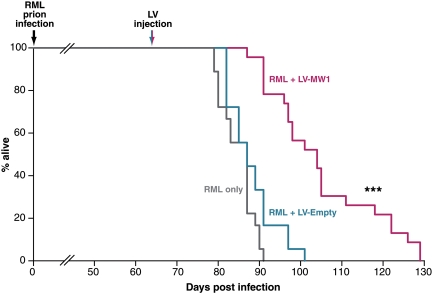
We assessed the effect of focal lentiviral treatment on survival time and examined neuronal integrity and spongiosis neuropathologically at time of death. We found a marked effect of treatment on survival. Even after a localized hippocampal injection, prion-infected mice treated with LV-MW1 survived significantly longer than mice treated with LV-Empty or those that received no virus (Fig. 3). Lentivirally mediated RNAi of PrP in the hippocampus resulted in survival of up to 129 days after infection (mean 105 ± 4 days), compared with mice treated with LV-Empty (mean 88 ± 3 days; P = 1.16E−05, Student's t test, two tails) or with no virus (mean 85 ± 3 days; P = 2.15E−07, Student's t test, two tails). This prolonged survival reflects mean incubation times ≈23.5% longer in LV-MW1-treated mice compared with untreated prion-infected tg37 mice and 19.3% longer than LV-Empty-treated mice, after one focal treatment with virus. (The difference in incubation times between LV-Empty-treated and untreated prion-infected mice was not significant: P = 0.067, Student's t test, two tails.)
Fig. 3.
Treatment with anti-PrP shRNA expressing lentivirus prolongs survival in mice with established prion disease. Mice were infected with RML prions at 1 week of age and were treated with bilateral hippocampal injections of either LV-MW1 (n = 22) or LV-Empty (n = 18) at 8 wpi or with no virus (n = 18). RML-infected mice treated with no virus or with LV-Empty died within 91 and 101 days postinfection (dpi), respectively; mice treated with LV-MW1 survived longer, living up to 129 dpi. (P < 0.0001 Student's t test, two tails, compared with both LV-Empty-treated and untreated mice).
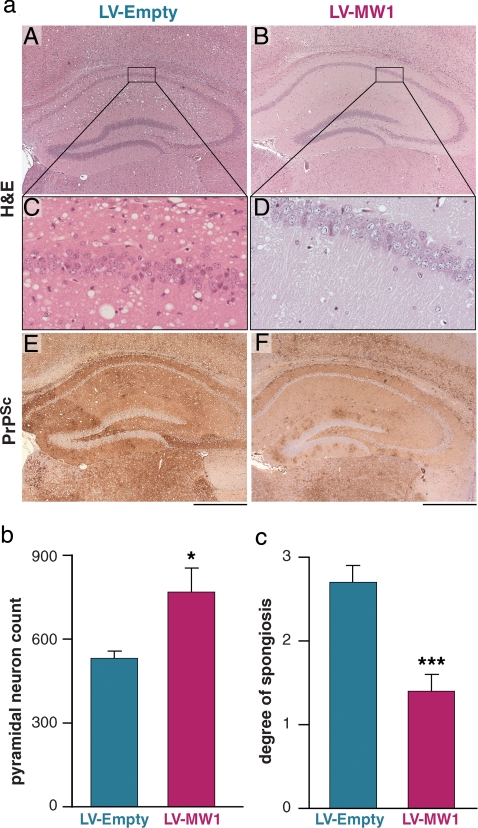
Neuropathological examination was performed blind on up to nine brains from each group of mice, selected at random, culled when they developed diagnostic clinical signs of terminal prion disease. Brain slices were assessed morphologically, scored for spongiosis and PrPSc deposition, and systematic neuronal counts of hippocampal CA1 neurons were performed. We found that RNAi of PrP expression in prion-infected mice was both focally neuroprotective in the hippocampus, and to a lesser degree more extensively throughout the brain. LV-MW1 treatment protected against hippocampal neuronal loss, which was apparent histologically (Fig. 4aD). Systematic neuronal counts confirmed preservation of CA1 pyramidal cell numbers in LV-MW1-treated animals: 852 ± 85 neurons compared with 532 ± 24 neurons (P = 0.029, Student's t test, two tails) in LV-Empty-treated mice (Fig. 4b). Hippocampal spongiform degeneration was also significantly less extensive in LV-MW1-treated mice (Fig. 4a B and D) compared to LV-Empty mice (Fig. 4a A and C). This was confirmed by blind semiquantitative scoring of spongiosis using a scale of 0–3 [indicating a range of absent (0) to severe (3) spongiosis] (20). Animals with RNAi-mediated PrP knockdown (n = 9) had a mean spongiosis score of 1.3 ± 0.2 in the hippocampus, representing mild spongiosis, compared with a score of 2.7 ± 0.2 (n = 6) in LV-Empty-treated mice, consistent with severe spongiform degeneration (P = 0.0007, Student's t test, two tails) (Fig. 4 c and Table S1). Hippocampal PrPSc accumulation was also reduced in LV-MW1-treated mice compared with LV-E-treated animals (Fig. 4a E and F and Table S1), even though LV-MW1-treated mice were on average culled later because of their overall longer survival, allowing more time for prion replication and PrPSc accumulation in nontransduced cells in these mice.
Fig. 4.
Lentivirally mediated RNAi of PrP expression protects against prion neurodegeneration in vivo. (a) LV-MW1 treatment protected against neuronal loss and reduced spongiform degeneration in the CA1–3 region (B and D) compared with LV-Empty-treated mice where neuronal loss is evident and spongiosis is marked (A and C). (The right hippocampus is shown for all mice.) PrPSc deposition was reduced in LV-MW1-treated mice (F) but was extensive in LV-Empty-treated animals (E). (Scale bar, 500 μm.) (b) Systematic counting of pyramidal cell neurons in CA1 confirmed neuroprotective effects of LV-MW1 (P = 0.029, Student's t test, two tails) and (c) semiquantitative scoring of spongiosis showed significantly less spongiosis in LV-MW1- treated mice (P = 0.0007, Student's t test, two tails) (n = 6 in each case).
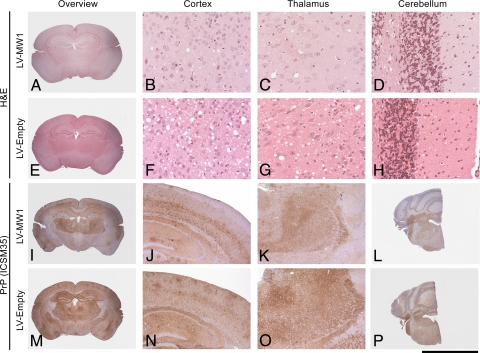
Evaluation of whole-brain pathology in LV-MW1-treated animals further showed reduction of spongiosis and PrPSc deposition beyond the targeted hippocampal region, in thalamus and cortex (but not in cerebellum), compared with LV-Empty-treated mice (Fig. 5 and Table S1). The morphological differences seen were statistically significant in the thalamus but not in the cortex (P = 0.03 for spongiosis and P = 0.04 for PrPSc deposition in the thalamus, Student's t test, two tails, unequal variance). This reduced pathology likely reflects altered prion spread and replication in these areas after hippocampal PrP knockdown by lentiviral injection, rather than more widespread PrP depletion arising from spread of virus. We did not find evidence of lentiviral expression in tissues beyond the injected hippocampi using immunohistochemistry for lentiviral EGFP expression. Further, unilateral injections of LV-MW1 into the right hippocampus of prion-infected mice reduced PrP mRNA levels only on this side, but not in the closely connected left hippocampus, injected with LV-Empty, where levels were essentially unchanged (Fig. S2).
Fig. 5.
Focal delivery of lentivirally mediated RNAi of PrP expression to hippocampus reduces spongiosis and PrPSc deposition in other brain regions. Representative sections from various brain regions from prion-infected mice treated with bilateral hippocampal injections of LV-MW1 8 weeks after infection (A–D and I–L) and LV-Empty (E–H and M–P). Sections are stained with H&E to show spongiosis (top two rows) and ICSM35 for PrPSc deposition (bottom two rows). LV-MW1 treatment reduces prion spongiosis and PrPSc accumulation in thalamus and cortex and in the hippocampus. [Scale bar, 5 mm (A, E, I, M), 4 mm (L and P), 1.3 mm (J, K, N, O), and 170 μm (B–D, F–H).]
Discussion
The rescue of early neuronal dysfunction before neuronal loss is established is a clear goal for therapeutic intervention in neurodegenerative disease. Our previous findings that transgene-mediated PrP knockdown reversed predegenerative pathological changes and early behavioral deficits in prion disease led us to try to achieve the same effect therapeutically. PrPC knockout, both during development and postnatally, appears to be without detrimental effect (6, 21). We used RNAi to silence PrP expression in mice with established prion disease. Knockdown of PrP by RNAi (11) and resultant inhibition of PrPSc replication in cell culture have been described (12), and RNAi of PrP also works in vivo. Transgenic mice generated by lentiviral transduction of embryos stably express anti-PrP shRNAs and have increased resistance to prion infection because of RNAi-mediated reduced expression of endogenous PrP (13). However, until now, RNAi had not been used therapeutically in vivo in prion disease.
Here, we have shown that treating mice with lentiviruses expressing shRNAs to knockdown PrP in established prion disease rescued early neuronal dysfunction and death in targeted areas and significantly prolonged survival. Injection of virus into the hippocampus 8 weeks after prion infection prevented the first behavioral deficits associated with early pathology of the CA1 region: loss of burrowing activity and object recognition memory (22) (Fig. 2). In our previous work, where PrP knockdown was due to recombination at the genomic level at ≈8 wpi, early deficits occurred but recovered rapidly in PrP-depleted animals. Here, injection of lentivirus expressing anti-PrP shRNAs at 8 wpi prevented their manifestation altogether, perhaps because posttranscriptional gene silencing is more rapid, or more tightly controlled temporally, than genetic excision of PrP encoding sequences after transgene expression.
The benefits of RNAi treatment were also seen morphologically. There was significantly less spongiform degeneration and neuronal loss where anti-PrP lentivirus was delivered. These changes progress rapidly in RML-infected tg37 mice after 8 wpi, particularly in the hippocampus (4), and were marked in terminally ill LV-Empty-treated animals at ≈12 wpi (Fig. 4). However, LV-MW1-treated mice culled up to 3 weeks later had minimal hippocampal spongiform change and neuronal loss (Fig. 4), suggesting sustained focal protection against neurotoxicity where PrP knockdown occurred. Interestingly, spongiosis was also reduced, although less significantly, in thalamus and cortex of animals treated with hippocampal injections (Fig. 5). PrPSc accumulation was also lower in animals with virally mediated RNAi of PrP in the hippocampus than in mock treated animals, and again this reduction was seen beyond the hippocampus, in thalamus and cortex. The more widespread changes are likely to reflect altered spread of prion infection after hippocampal PrP knockdown, as discussed below. Of note, PrPSc accumulation did not appear to affect neuronal function or survival, as reflected in preservation of hippocampal behaviors and structural neuronal integrity, and consistent with our observations in mice with Cre-mediated PrP depletion (5), which has implications for the level of knockdown required for therapeutic effect. Thus, simply slowing the rate of prion replication, here by reducing PrPC levels, may be effective for prevention of neurotoxic effects.
The critical effect, however, was the effect of this treatment on survival of prion-infected mice. A single treatment with focal injection of virus resulted in significantly prolonged survival time of treated animals, compared with mock or untreated mice, with a mean increase in lifespan of 23.5% compared with untreated animals (Fig. 3). The spread of incubation times in the LV-MW1 group (87–129 days after inoculation, mean 105 ± 4 days) is probably due to variation in neuronal transduction by virus seen in individual mice (data not shown) or variability between individual injections, with the highest levels of transduction affording the greatest protection and longest survival. The increased survival was strikingly large with respect to the very small volume of brain targeted. This may result from direct or indirect effects of localized neuroprotection or may simply be due to the reduction of PrP expression at a critical, or rate-limiting, site for prion replication. Prion incubation times are known to be inversely proportional to overall levels of PrP expression (3, 23, 24), and it is likely that regional variations also affect prion replication rates and incubation periods. The hippocampus is a focus of early prion replication and PrPSc deposition (see Fig. S3) both for RML and other prion strains in various inbred lines and in some transgenic mice (25, 26), including tg37 mice, used here (4). We showed up to 80% reduction of hippocampal PrP mRNA expression (Fig. 1B) with single LV-MW1 administration; this localized knockdown may therefore eliminate a key area for early prion replication in this model. Further, we have found no evidence for the spread of lentivirus beyond the injection site, supporting the concept that it is the effect of localized hippocampal PrP depletion that alters the spread and replication of RML prions in this model. Clearly, all animals succumb eventually, presumably due to prion-mediated neurodegeneration in other critical brain regions, but the neuroprotective effects seen within the hippocampus and beyond are clearly a desirable effect of therapy. If transduction were to be more widespread, by pseudotyping lentiviruses with coat proteins that allow retrograde transport (27, 28) or using evolving mechanical techniques for enhanced delivery (29–31), more extensive neuroprotection and longer survival might ensue. Even focal targeting may have therapeutic application in some situations, however.
In conclusion, we have used lentivirally mediated RNAi for treatment of established prion infection in mice. Even localized single administration of these viruses to the hippocampus prolonged the lifespan of infected mice, protected transduced neurons from degenerating, reduced PrPSc accumulation, and prevented the onset of the first behavioral deficits associated with the disease. Our findings further support therapeutic strategies directed at PrP knockdown for the treatment of prion diseases and are also relevant for neurodegeneration more widely, highlighting the importance of intervention when neuronal dysfunction can still be reversed. The approach used here paves the way not only for possible future therapy but also for mechanistic dissection of toxicity and recovery in prion diseases. Further exploration of the extent and timing of RNAi-mediated PrP knockdown required for increased therapeutic effect in prion disease can now be undertaken.
Methods
shRNA-Expressing Lentivirus Production.
We generated shRNAs, corresponding to siRNA sequences known to knock down PrP in vitro (described in SI Text). These were synthesized as a 21-nt inverse repeat separated by a 9-nt loop for each sequence (MWG Biotech) (see SI Text and Fig. 1) and inserted downstream of the U6 promoter in the lentiviral vector pLL3.7 (14). Lentiviruses were generated by triple transfection of ≈80% confluent HEK293FT cells (Invitrogen) with modified pLL3.7 plasmid and pCMV_dR8_74 and pMD2 helper plasmids (gifts of Tronolab) using FuGENE6 transfection reagent (Roche), as described (14). Lentiviruses were harvested in serum-free medium after 3 days, filtered and concentrated in primed Centricon Plus-20 filter devices (Millipore). Titer was determined as described in SI Text. Additional stocks of virus were produced by the Lentiviral Vector Production Unit (Swiss Institute of Technology, Lausanne, Switzerland) supported by the Association Française Contre les Myopathies (AFM).
Viral Transduction.
Neuronal cell lines were transduced with virus at multiplicities of infection ranging from 1 to 10 by addition of concentrated virus to the culture media. Twenty-four hours later, the media were replaced, and the cells were monitored for EGFP expression.
Immunocytochemical Detection of PrP in Lentivirally Transduced Cells.
Cells (N2a or GT-1; see SI Text) were fixed on coverslips and immunostained with primary antibody ICSM18 (DGen) at 10 μg/ml 4 days after viral transduction. Secondary antibody was Alexa Fluor 568 goat anti-mouse IgG (H+L) (Molecular Probes) at 1:800 dilution. DAKO fluorescent mounting medium containing 1 μg/ml DAPI (Sigma) was used for mounting coverslips. Cells were viewed on an Axioplan 2 MOT microscope (Zeiss) with an AxioCam MRm camera and Axiovision Control software (Zeiss).
RNA Extraction.
RNA was extracted from cell/tissue homogenate with an RNeasy midi kit (Qiagen). Harvested cells (1 × 107) were homogenized in Buffer RLT (Qiagen) with a 20-gauge needle. Freshly dissected whole hippocampi were homogenized in a ribolyzer (Hybaid) at 6.5 M per sec for 45 sec using ceramic beads in buffer RLT as above.
RT-PCR.
One-step RT-PCR amplification of endogenous PrP mRNA or of the MloxP transgene mRNA, and β-actin was performed on 25 ng of RNA using a Quantitect Multiplex kit (Qiagen) with ROX reference dye on a PRISM 7000 Taqman machine (Applied Biosystems). (For primers, probes and cycling conditions see SI Text). All reactions were performed in triplicate and negative controls included H2O only and omission of reverse transcriptase. PrP expression was normalized to β-actin.
Animal Work.
All animal work conformed to United Kingdom regulations and institutional guidelines and was performed under Home Office project license. tg37 transgenic mice were bred in-house and were housed in a temperature and light-controlled mouse colony room (12-h light/dark cycle) in groups of four to six. All mice had free access to food and water. The strain genetic background is predominantly FVB after 10 generations of back-crossing. All animals are hemizygous for the MloxP transgene.
Inoculation with RML prions was performed as described (4). Animals were culled when they developed diagnostic clinical signs of terminal prion disease.
Stereotaxic Injection.
Mice were anesthetized with 100 mg/kg ketamine (Amersham Pharmacia and Upjohn) + 10 mg/kg xylazine (Bayer PLC). Two microliters of virus containing 2 × 106 transducing units was injected at 2 mm posterior to bregma, 1.5–3 mm lateral and 1.8–2 mm ventral, using a 26-gauge 700 series needle and Hamilton syringe (Hamilton) at 0.5 μl per minute.
Behavioral Testing.
All testing took place in a room with somber lighting and constant background noise. Mice were handled for 10 min per day for several days and habituated to test environment before testing. Only group-housed animals were used. Groups of ≈20 mice were tested sequentially over time for each paradigm, unless otherwise stated. Mice were handled, habituated, trained, and tested at the same time for each experiment. Object recognition (32) and burrowing tasks (16) were performed as described (5); see also SI Text.
CA1 Region Pyramidal Cell Counts.
We used a Zeiss Axioskop microscope with motorized stage linked to an image-analysis system (Histometrix 6; Kinetic Imaging Limited). This allows automated 2D analyses and intensity labeling of features in RGB (red-green-blue) images for automatic particle identification. A video camera attached to the microscope provided live images for the analyses. The XY and Z motorized stage was used to outline regions of interest larger than a single field of view and was used to sample the entire CA1 region. At least six mice treated with each virus combination were analyzed, and regression was calculated.
Neuropathological Examination of Brain Sections.
PrPSc was detected using monoclonal antibody to PrP, ICSM35 (DGen), using an automated immunostaining system (Ventanamed), as described (6).
Statistical Analyses.
These were performed in Microsoft Excel and GraphPad InStat Version 3.05 (GraphPad Software). Individual datasets were tested for normality by using the one-sample Kolmogorov–Smirnov test. Student's t tests were applied to all datasets with two tails (two samples; unequal variance).
Supplementary Material
Acknowledgments.
We thank C. O'Malley, C. Powell, and J. Underwood for technical assistance; R.Young for help with graphics; and Maria Thom for use of and training in the Zeiss Axioskop microscope. This work was funded by the Medical Research Council, Great Britain.
Footnotes
Conflict of interest statement: J.C. is a director and shareholder of D-Gen Limited, an academic spin-out company working in the field of prion disease diagnosis, decontamination, and therapeutics. D-Gen markets the ICSM35 and ICS18 antibodies used in this study.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802759105/DCSupplemental.
References
- 1.Bueler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Sailer A, et al. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 3.Manson JC, et al. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 4.Mallucci G, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 5.Mallucci GR, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Mallucci G, et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 2002;21:202–210. doi: 10.1093/emboj/21.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Alegre P, Bode N, Davidson BL, Paulson HL. Silencing primary dystonia: lentiviral-mediated RNA interference therapy for DYT1 dystonia. J Neurosci. 2005;25:10502–10509. doi: 10.1523/JNEUROSCI.3016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer O, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 9.Xia H, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 10.Sapru MK, et al. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Tilly G, et al. Efficient and specific down-regulation of prion protein expression by RNAi. Biochem Biophys Res Commun. 2003;305:548–551. doi: 10.1016/s0006-291x(03)00805-2. [DOI] [PubMed] [Google Scholar]
- 12.Daude N, Marella M, Chabry J. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J Cell Sci. 2003;116:2775–2779. doi: 10.1242/jcs.00494. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer A, et al. Lentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected mice. J Clin Invest. 2006;116:3204–3210. doi: 10.1172/JCI29236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinson DA, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 15.Duale H, Kasparov S, Paton JF, Teschemacher AG. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp Physiol. 2005;90:71–78. doi: 10.1113/expphysiol.2004.029173. [DOI] [PubMed] [Google Scholar]
- 16.Deacon RMJ, Raley JM, Perry VH, Rawlins JNP. Burrowing into prion disease. NeuroReport. 2001;12:2053–2057. doi: 10.1097/00001756-200107030-00052. [DOI] [PubMed] [Google Scholar]
- 17.Betmouni S, Deacon RMJ, Rawlins JNP, Ferry VH. Behavioral consequences of prion disease targeted to the hippocampus in a mouse model of scrapie. Psychobiology. 1999;27:63–71. [Google Scholar]
- 18.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 20.Bruce ME, McBride PA, Farquhar CF. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989;102:1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- 21.Bueler H, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham C, et al. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- 23.Bueler H, et al. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer M, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 25.Scott M, et al. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993;73:979–988. doi: 10.1016/0092-8674(93)90275-u. [DOI] [PubMed] [Google Scholar]
- 26.Brown DA, Bruce ME, Fraser JR. Comparison of the neuropathological characteristics of bovine spongiform encephalopathy (BSE) and variant Creutzfeldt-Jakob disease (vCJD) in mice. Neuropathol Appl Neurobiol. 2003;29:262–272. doi: 10.1046/j.1365-2990.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 27.Kato S, et al. Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein. Hum Gene Ther. 2007;18:1141–1151. doi: 10.1089/hum.2007.082. [DOI] [PubMed] [Google Scholar]
- 28.Mazarakis ND, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Neurosci Res. 2007;57:550–558. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 29.Hadaczek P, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, et al. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J Neurosurg. 2007;107:568–577. doi: 10.3171/JNS-07/09/0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanftner LM, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol. 2005;194:476–483. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.