Abstract
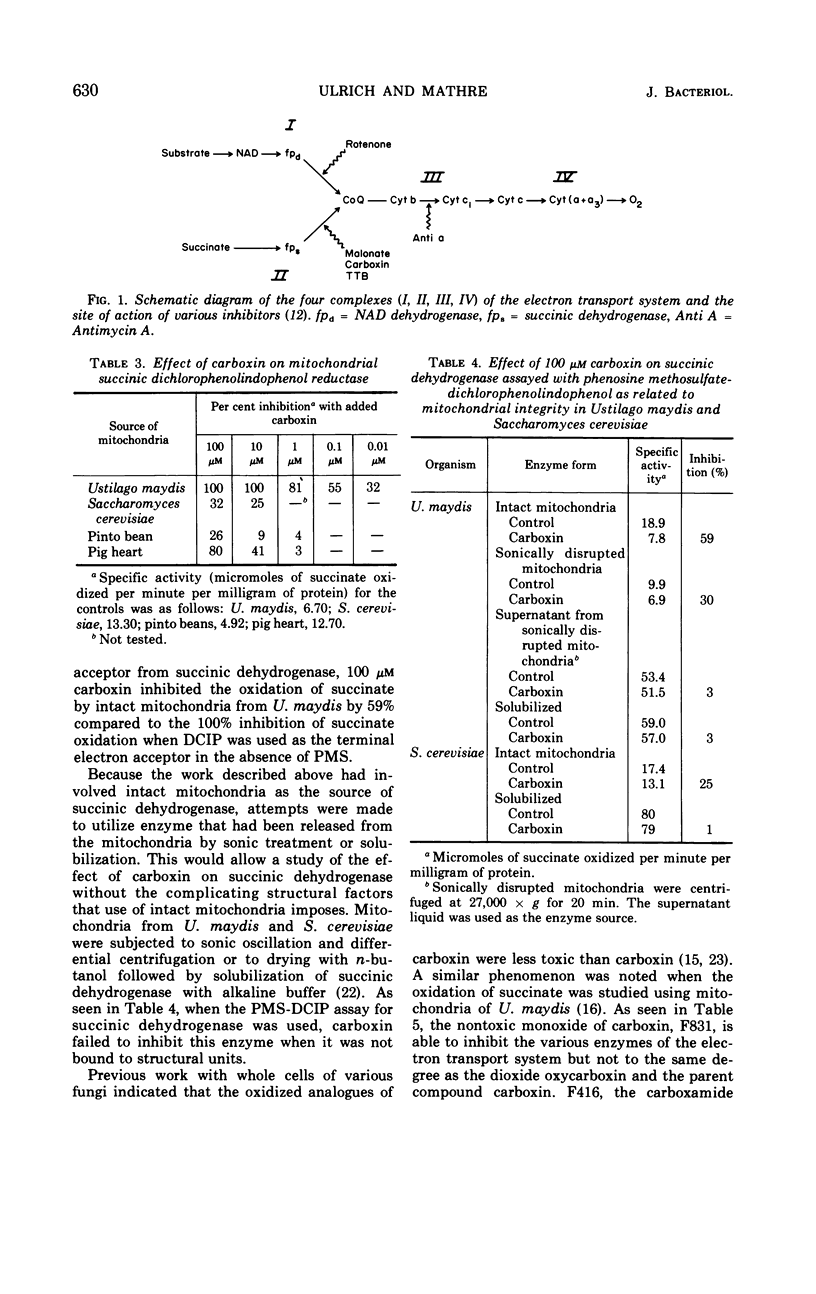
The systemic fungicide carboxin (5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide) at 100 μm inhibited succinate cytochrome c reductase in mitochondria from Ustilago maydis and Saccharomyces cerevisiae. It did not have any effect on reduced nicotinamide adenine dinucleotide (NADH) cytochrome c reductase. Succinate coenzyme Q reductase was also inhibited, but NADH coenzyme Q reductase was not. When dichlorophenolindophenol (DCIP) was used as the terminal acceptor of electrons from the oxidation of succinate, carboxin was very effective in inhibiting succinate-DCIP reductase. Carboxin was inhibitory to succinic dehydrogenase assayed with phenazine methosulfate plus DCIP when intact mitochondria were used as the enzyme source but not when solubilized enzyme was used. The main site of action of carboxin, therefore, appears to lie between succinate and coenzyme Q. The dioxide analogue of carboxin was also effective in inhibiting succinate-cytochrome c reductase, succinate-coenzyme Q reductase, or succinate-DCIP reductase, whereas the monoxide analogue was less effective in inhibiting these enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Gottlieb D. Mechanism of action of the fungicide thiabendazole, 2-(4'-thiazolyl) benzimidazole. Appl Microbiol. 1970 Dec;20(6):919–926. doi: 10.1128/am.20.6.919-926.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Edgington L. V., Barron G. L. Fungitoxic spectrum of oxathiin compounds. Phytopathology. 1967 Nov;57(11):1256–1257. [PubMed] [Google Scholar]
- Edgington L. V., Walton G. S., Miller P. M. Fungicide selective for basidiomycetes. Science. 1966 Jul 15;153(3733):307–308. doi: 10.1126/science.153.3733.307. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathre D. E. Mode of action of oxathiin systemic fungicides. I. Effect of carboxin and oxycarboxin on the general metabolism of several basidiomycetes. Phytopathology. 1970 Apr;60(4):671–676. doi: 10.1094/phyto-60-671. [DOI] [PubMed] [Google Scholar]
- RAMASARMA T., LESTER R. L. Studies on the electron transport system. XXIV. The reduction and oxidation of exogenous coenzyme Q. J Biol Chem. 1960 Nov;235:3309–3314. [PubMed] [Google Scholar]
- Ragsdale N. N., Sisler H. D. Metabolic effects related to fungitoxicity of carboxin. Phytopathology. 1970 Oct;60(10):1422–1427. doi: 10.1094/phyto-60-1422. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., MASSEY V., KEARNEY E. B. Studies on succinic dehydrogenase. V. Isolation and properties of the dehydrogenase from baker's yeast. Arch Biochem Biophys. 1957 Jul;69:405–421. doi: 10.1016/0003-9861(57)90506-4. [DOI] [PubMed] [Google Scholar]
- Schmeling B. V., Kulka M. Systemic fungicidal activity of 1,4-oxathiin derivatives. Science. 1966 Apr 29;152(3722):659–660. doi: 10.1126/science.152.3722.659. [DOI] [PubMed] [Google Scholar]
- Sherald J. L., Sisler H. D. Antimycin A-resistant respiratory pathway in Ustilago maydis and Neurospora sitophila. Plant Physiol. 1970 Jul;46(1):180–182. doi: 10.1104/pp.46.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel M., von Schmeling B., Edgington L. V. Fungitoxicity and structure-activity relationships of some oxathiin and thiazole derivatives. Phytopathology. 1970 Aug;60(8):1164–1169. doi: 10.1094/phyto-60-1164. [DOI] [PubMed] [Google Scholar]
- Tripathi R. K., Gottlieb D. Mechanism of action of the antifungal antibiotic pyrrolnitrin. J Bacteriol. 1969 Oct;100(1):310–318. doi: 10.1128/jb.100.1.310-318.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. A. A potent effect of 1,4-oxathiin systemic fungicides on succinate oxidation by a particulate preparation from Ustilago maydis. Biochem Biophys Res Commun. 1971 Sep;44(5):1212–1219. doi: 10.1016/s0006-291x(71)80215-2. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Airall J. M. The mode of action of antifungal agents: effect of pyrrolnitrin on mitochondrial electron transport. J Antibiot (Tokyo) 1970 Feb;23(2):55–62. doi: 10.7164/antibiotics.23.55. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Horng J. S., Gordee R. S. Respiratory chain of a pathogenic fungus, Microsporum gypseum: effect of the antifungal agent pyrrolnitrin. J Bacteriol. 1971 Apr;106(1):168–173. doi: 10.1128/jb.106.1.168-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


