Abstract
Emerging evidence suggests that dietary phytochemicals, in particular flavonoids, may exert beneficial effects in the central nervous system by protecting neurons against stress-induced injury, by suppressing neuroinflammation and by promoting neurocognitive performance, through changes in synaptic plasticity. It is likely that flavonoids exert such effects in neurons, through selective actions on different components within a number of protein kinase and lipid kinase signalling cascades, such as phosphatidylinositol-3 kinase (PI3K)/Akt, protein kinase C and mitogen-activated protein kinase. This review details the potential inhibitory or stimulatory actions of flavonoids within these pathways, and describes how such interactions are likely to affect cellular function through changes in the activation state of target molecules and/or by modulating gene expression. Although, precise sites of action are presently unknown, their abilities to: (1) bind to ATP binding sites on enzymes and receptors; (2) modulate the activity of kinases directly; (3) affect the function of important phosphatases; (4) preserve neuronal Ca2+ homeostasis; and (5) modulate signalling cascades lying downstream of kinases, are explored. Future research directions are outlined in relation to their precise site(s) of action within the signalling pathways and the sequence of events that allow them to regulate neuronal function in the central nervous system.
Keywords: Flavonoid, Neuron, MAPK, ERK, JNK, Akt, PI3K, Neurodegeneration
Introduction
Recently, there has been intense interest in the potential of flavonoids to modulate neuronal function and prevent age-related neurodegeneration. Dietary supplementation studies, in humans and animals, using flavonoid-rich plant or food extracts have highlighted their potential to influence cognition and learning [60, 70, 95, 175, 183, 194, 197], presumably by protecting vulnerable neurons, enhancing existing neuronal function or by stimulating neuronal regeneration. Their neuroprotective potential has been shown in both oxidative stress- [77] and Aβ-induced-neuronal death models [109]. Evidence also supports the beneficial and neuromodulatory effects of flavonoid-rich ginkgo biloba extracts, particularly in connection with age-related dementias and Alzheimer’s disease [14, 109, 203]. Furthermore, the citrus flavanone, tangeretin, has been observed to maintain nigrostriatal integrity and functionality following lesioning with 6-hydroxydopamine, suggesting that it may serve as a potential neuroprotective agent against the underlying pathology associated with Parkinson’s disease [42]. The exact mechanisms by which flavonoids might exert such effects are not fully understood, although evidence from cell studies suggests that flavonoids express a wide variety of cellular actions and may influence neuronal function via the modulation of critical neuronal signalling pathways.
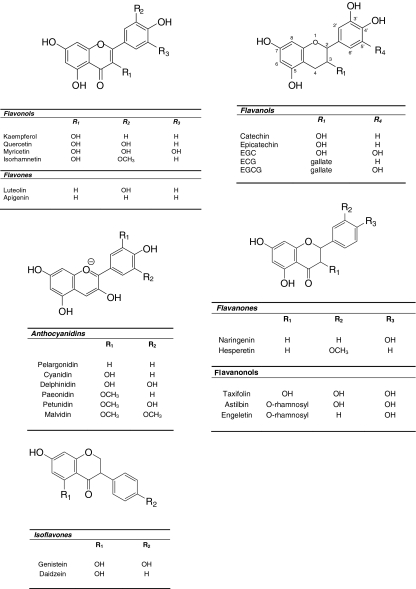
Flavonoids comprise the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants. Major dietary sources of flavonoids include fruits, vegetables, tea, wine, cereals and fruit juices (reviewed in [111]). Flavonoids consist of two aromatic carbon rings: benzopyran (A and C rings) and a benzene (B ring) (Fig. 1) and may be divided in six subgroups based on the degree of the oxidation of the C-ring, the hydroxylation pattern of the ring structure and the substitution of the 3-position. The main dietary groups of flavonoids are (1) flavonols (e.g. kaempferol, quercetin), which are found in onions, leeks, broccoli, (2) flavones (e.g. apigenin, luteolin), which are found in parsley and celery, (3) isoflavones (e.g. daidzein, genistein), which are mainly found in soy and soy products, (4) flavanones (e.g. hesperetin, naringenin), which are mainly found in citrus fruit and tomatoes, (5) flavanols [e.g. catechin, epicatechin, epigallocatechin, epigallocatechin gallate (EGCG)], which are abundant in green tea, red wine, chocolate, and (6) anthocyanidins (e.g. pelargonidin, cyanidin, malvidin), whose sources include red wine and berry fruits. Further information regarding the structure and classes of flavonoids may be found in the thorough review by Manach et al. [111].
Fig. 1.
The structures of the five main classes of flavonoids. The major differences between the individual groups reside in the hydroxylation pattern of the ring-structure, the degree of saturation of the C-ring and the substitution of the 3-position
Historically, the biological actions of flavonoids have been attributed to their antioxidant properties, either through their reducing capacities per se or through their influences on the intracellular redox status [139, 140]. However, it has been speculated that their classical hydrogen donating antioxidant activity is not the explanation for the bioactivity of flavonoids in vivo. Indeed, it has become evident that flavonoids are more likely to exert their neuroprotective actions by (1) the modulation of intracellular signalling cascades which control neuronal survival, death and differentiation; (2) affecting gene expression and (3) interactions with mitochondria [148, 159, 185]. This review will highlight some of the interactions of flavonoids with major neuronal intracellular signalling pathways, in particular those that are vital in determining neuronal death, survival, differentiation and proliferation. Although, there have been a huge number of investigations into the actions of flavonoids within signalling pathways, the aim of this review will be to highlight the potential mechanisms by which flavonoids interact within neuronal signalling cascades.
Flavonoid metabolism and access to the brain
Flavonoids display potent antioxidant capacity in vitro [138–140]. However, during absorption flavonoids are extensively metabolised resulting in a significant alteration in their redox potentials. It has become clear that the bioactive forms of flavonoids in vivo are not those forms found in plants. For example, the majority of flavonoid glycosides, and in some instances the aglycones, present in plant-derived foods, are extensively conjugated and metabolised during absorption (reviewed in [47, 51, 162, 163, 180]). In particular, there is much evidence for the extensive phase I de-glycosylation and phase II metabolism of the resulting aglycones to glucuronides, sulphates and O-methylated forms during transfer across the small intestine [157, 162] and then again in the liver. Further transformation has been reported in the colon where the enzymes of the gut microflora degrade flavonoids to simple phenolic acids [146]. In addition, flavonoids may undergo at least three types of intracellular metabolism: (1) Oxidative metabolism, (2) P450-related metabolism and (3) conjugation with thiols, particularly GSH [158, 160]. Circulating metabolites of flavonoids, such as glucuronides, sulphates and conjugated O-methylated forms, or intracellular metabolites like flavonoid-GSH adducts, have greatly reduced antioxidant potential [160]. Indeed, studies have indicated that although such conjugates and metabolites may participate directly in plasma antioxidant reactions and in scavenging reactive oxygen and nitrogen species in the circulation, their effectiveness is reduced relative to their parent aglycones [41, 122, 152, 170, 189].
In order to understand whether flavonoids and their metabolic derivatives are capable of direct neuroprotective effects, it is crucial to ascertain whether they are able to access the central nervous system. In order for flavonoids to enter into the brain, they must first cross the blood brain barrier (BBB). The function of the BBB includes protection of the brain from xenobiotics and the general maintenance of the brain’s microenvironment [2, 193]. Studies by Youdim et al. indicated that certain flavonoids were able to penetrate the BBB in relevant in vitro and in situ models [193, 195, 196]. In these studies, the flavanones, hesperetin, naringenin and their relevant in vivo metabolites, as well as some dietary anthocyanins, cyanidin-3-rutinoside and pelargonidin-3-glucoside, were able to traverse the BBB. Furthermore, it was demonstrated that the uptake of the relatively lipophilic flavanones, naringenin and hesperetin, was much greater than for other flavonoids, such as epicatechin, epicatechin metabolites, anthocyanins and their glucuronidated metabolites, which are more polar in nature. This study suggested that the potential for flavonoid penetration is dependent on compound lipophilicity [193]. Accordingly, it is plausible that the uptake of the less polar methylated metabolites, such as the methylated epicatechin metabolites (formed in the small intestine and liver), may be greater than the parent aglycone. For the same reason, the more polar flavonoid glucuronidated metabolites, which seem to have low BBB permeability values [193], may not be able to access the brain. However, evidence exists to suggest that certain drug glucuronides may cross the BBB [1] and exert pharmacological effects [94, 164], suggesting that there may be a specific uptake mechanism for glucuronides in vivo. It has also been suggested that apart from the components’ lipophilicity, the ability of flavonoids to enter the brain may be influenced by their interactions with specific efflux transporters expressed in the BBB. One such transporter is P-glycoprotein [195], which plays an important role in drug absorption and brain uptake [107]. In the study conducted by Youdim et al. [195], the differences between naringenin and quercetin flux into the brain in situ was attributed not only to their differences in lipophilicity, but also to their ability to act as efflux transporter substrates.
There is also evidence from animal feeding studies to suggest that flavonoids may access the brain. The tea flavanol EGCG has been reported to access the brain after oral administration to mice [166]. Furthermore, oral ingestion of pure epicatechin resulted in the detection of epicatechin glucuronide and 3′-O-methyl-epicatechin glucuronide in rat brain tissue [3]. Anthocyanidins have also been detected in the brain after oral administration [53, 168], with several anthocyanidins being identified in different regions of rat brain after the animals were fed with blueberry [12]. Such flavonoid localisation has been correlated with increased cognitive performance, suggesting a central neuroprotective role of these components.
It is clear that the concentrations of flavonoids and their metabolite forms accumulated in vivo, for example in the plasma or in organs such as the brain [3] are lower (high nM, low μM) than those recorded for small molecule antioxidant nutrients such as ascorbic acid and α-tocopherol [67]. In addition, these in vivo forms will mainly be metabolites possessing lower antioxidant potential relevant to parent compounds. Therefore, the beneficial effects of flavonoid metabolites in vivo are unlikely to result by their ability to out-compete antioxidants such as ascorbate, which are present at higher concentrations (high μM to mM). For example, flavonoids have been shown to protect neurons against oxidative stress more effectively than ascorbate even when the latter was used at tenfold higher concentrations [150], which supports a non-antioxidant mechanism of action. If such an antioxidant mechanism is unlikely in vivo, there must be other potential routes by which they exert beneficial effects at the cellular and tissue level. Over the last 5–10 years evidence has accumulated to suggest that the cellular effects of flavonoids may be mediated by their interactions with specific proteins central to intracellular signalling cascades [148]. In the next section we highlight the potential for these polyphenols and their metabolites to interact at various points within the mitogen activated protein kinase (MAP kinase) signalling pathway and the phosphoinositide 3-kinase (PI3 kinase/Akt) signalling cascade.
Flavonoid interactions with neuronal signalling cascades
Flavonoids have been shown to exert modulatory effects in neurons through selective actions at different components of a number of protein kinase and lipid kinase signalling cascades, such as the PI3 kinase (PI3K)/Akt, tyrosine kinase, protein kinase C (PKC) and mitogen-activated protein kinase (MAP kinase) signalling pathways [7, 61, 93, 116, 149, 159, 178] (Fig. 2). Inhibitory or stimulatory actions at these pathways are likely to profoundly affect cellular function by altering the phosphorylation state of target molecules and/or by modulating gene expression. Although selective inhibitory actions at these kinase cascades may be beneficial in cancer, proliferative diseases, inflammation and neurodegeneration they could be detrimental during development particularly in the immature nervous system when protein and lipid kinase signalling regulates survival, synaptogenesis and neurite outgrowth. In the mature brain, post-mitotic neurones utilise MAP kinase and PI3K cascades in the regulation of key functions such as synaptic plasticity and memory formation [106, 167] (Fig. 2), thus flavonoid interactions within these pathways could have unpredictable outcomes and will be dependent both on the cell type and disease studied.
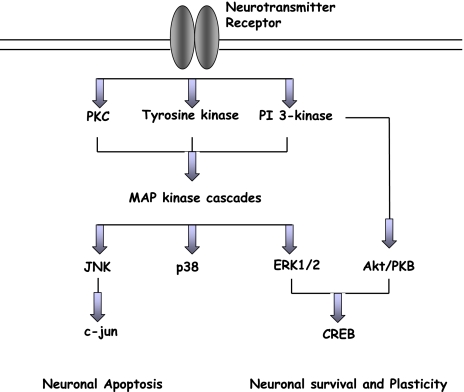
Fig. 2.
Overview of MAP kinase and Akt/PKB signalling cascades in neurons. Flavonoid-induced activation of ERK1/2 or PI3K/Akt pathways acts to stimulate neuronal survival and/or enhance synaptic plasticity and long-term potentiation relevant to the laying down of memory. In addition, inhibitory actions within JNK and p38 pathways are likely to be neuroprotective in the presence of stress
Flavonoids have the potential to bind to the ATP-binding sites of a large number of proteins [38] including, mitochondrial ATPase [50], calcium plasma membrane ATPase [13], protein kinase A [136], protein kinase C [61, 81, 101, 142, 176] and topoisomerase [18]. In addition, interactions with the benzodiazepine binding sites of GABA-A receptors and with adenosine receptors [48, 117] have been reported. For example, the stilbene resveratrol and the citrus flavanones, hesperetin and naringenin, have been reported to have inhibitory activity at a number of protein kinases [56, 73, 154]. This inhibition is mediated via the binding of the polyphenols to the ATP binding site, presumably causing three-dimensional structural changes in the kinase leading to its inactivity. They may also interact directly with mitochondria, for example, by modulating the mitochondrial transition pore (mPT), which controls cytochrome c release during apoptosis [65, 169], or by modulating other mitochondrial associated pro-apoptotic factors such as DIABLO/smac [64, 165]. Potential interactions with the mPT are especially interesting, as the transition pore possesses a benzodiazepine-binding site where flavonoids may bind [48, 117] and influence pore opening and cytochrome c release during apoptosis.
Flavonoids may also be capable of modulating glutamate excitotoxicity via direct scavenging of ROS or by the modulation of calcium influx. Abnormal influx of Ca2+ through AMPA-type glutamate receptors has been strongly implicated in neuronal death associated with a number of brain disorders through activation of Ca2+-dependent proteases, phospholipases and stress-activated kinases. Flavonoids may be capable of rendering heteromeric AMPA receptor assemblies Ca2+-impermeable by up-regulating GluR2 subunit expression [147]. Alternatively, flavonoids and their metabolites may prevent neuronal injury by scavenging of reactive intermediates such as superoxide and peroxynitrite derived from calcium mediated activation of xanthine oxidase and nitric oxide synthase, respectively. Lastly, modulation of signalling pathways and inhibition of calcium-activated kinases may also act to prevent excitotoxic death in neurons.
MAP kinase signalling cascade
Mitogen-activated protein kinases (MAPK) belong to the super-family of serine/threonine kinases and play a central role in transducing various extracellular signals into intracellular responses [36, 63]. MAPK cascades are organised into three main levels of regulation: (1) a MAP kinase kinase kinase (MAPKKK), which phosphorylates and activates (2) a MAP kinase kinase (MAPKK), which in turn, phosphorylates and activates (3) a MAPK [36, 114]. The best characterised MAPK pathways are the mitogenic extracellular signal-regulated protein kinase (ERK) pathway (Fig. 3) and the stress activated, c-Jun N-terminal kinase (JNK) (Fig. 4) and p38 (Fig. 5) cascades. Once activated, ERK, JNK and p38 phosphorylate a number of cytosolic proteins and transcription factors resulting in the enhancement of their transcriptional activities and activation of dependent genes [84].
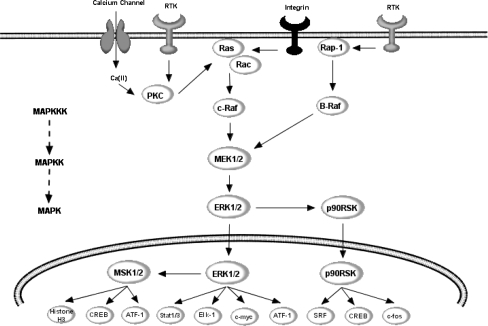
Fig. 3.
Potential points of action of flavonoids within ERK pathway. ERK1/2 are activated by upstream MAPKK, such as MEK1/2, and MAPKKK, such as c-Raf. MEK1/2 induce ERK1/2 activation via dual phosphorylation on threonine 202 and tyrosine 204 residues within the tripeptide motif TEY. Phosphorylation of ERK leads to the activation of a number of transcription factors, important in controlling differentiation, neuronal survival and various forms of cellular plasticity. For example, ERK activates pro-survival transcription factor CREB, by activating both p90RSK and MSK1/2. Other important targets include, ATF-1, Elk-1 and stat1/3, all of which are implicated in the regulation of various forms of cellular stress, including genotoxic agents, inflammatory cytokines and UV irradiation
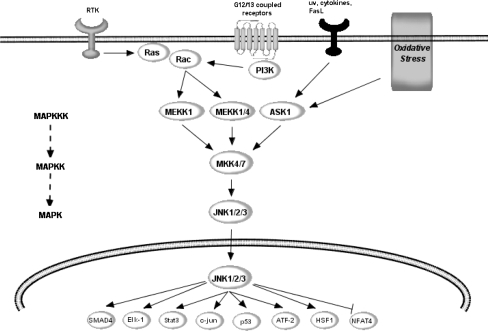
Fig. 4.
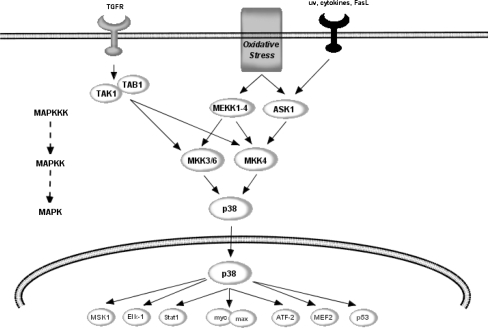
Potential points of action of flavonoids within JNK pathway. JNK is regulated by a variety of MAPKKK’s, including MEKK1/4 and apoptosis-stimulating kinase (ASK1), which is further regulated by GTPases and Rac1. Unlike the ERK pathway, the JNK cascade is strongly activated by stress signals such as UV and γ-radiation, oxidative stress and inflammatory cytokines. The MAPKK, MKK4/7, dually phosphorylates JNK within the Thr138-Pro-Tyr-185 motif (pTPpY) in the catalytic core of active JNK. Active JNK has been strongly linked to transcription-dependent apoptotic signalling, mainly through the activation of c-Jun and other AP-1 proteins including JunB, JunD and ATF-2
Fig. 5.
Potential points of action of flavonoids within p38 pathway. p38 is activated by a variety of cellular stresses including osmotic shock, pro-inflammatory inflammatory cytokines, lipopolysacharides, UV light and growth factors. The activity of p38 is regulated by the MKK’s, MKK3 and MKK6, which in turn, are regulated by the upstream MAPKKK, MEK1/4 and ASK. Active p38 regulates the phosphorylation of the transcription factors ATF-2, Max and MEF2, which are implicated in cellular response to a variety of cellular stress, including genotoxic agents and inflammatory cytokines
ERK and JNK are generally considered as having opposing actions, in particular in neuronal apoptosis [188]. ERK1/2 are usually associated with pro-survival signalling [11, 19, 83] through mechanisms that may involve activation of the cAMP response element binding protein (CREB) [19, 39] (Fig. 3), the up-regulation of the anti-apoptotic protein Bcl-2 and non-transcriptional inhibition of BAD [19, 83]. On the other hand, JNK has been strongly linked to transcription-dependent apoptotic signalling [118, 198], possibly through the activation of c-Jun [15] and other AP-1 proteins including JunB, JunD and ATF-2 [46] (Fig. 4). Many investigations have indicated that flavonoids and their metabolites may interact selectively within the MAPK signalling pathways [92, 93]. The potential modulation of MAPK signalling by flavonoids is significant as ERK1/2 and c-Jun amino-terminal kinase are involved in growth factor induced mitogenesis, differentiation, apoptosis and various forms of cellular plasticity [32, 33, 71, 118, 198].
Oxidative stress also has a diverse effect on the MAPK cascade [17, 172, 174]. Changes in the cellular redox status may result in the activation of pro-apoptotic signalling proteins such as JNK [46, 129, 141, 145, 148], which may initiate apoptotic mechanisms within cells [96] (Fig. 4). Additionally, oxidative stress may affect mitochondria by influencing the mitochondrial transition pore (mPT) and/or release of cytochrome c [88, 102]. There is strong evidence linking the activation of JNK to neuronal death in response to a wide array of pro-apoptotic stimuli both in developmental and degenerative death signalling [46, 118]. In the context of oxidative insults in neurons, JNK has been shown to be activated by dopamine [110], by 4-HNE [24, 132, 155], through reduced expression of SOD1 [113], by hydrogen peroxide [39] and by oxLDL [149].
Extracellular signal-regulated protein kinase pathway
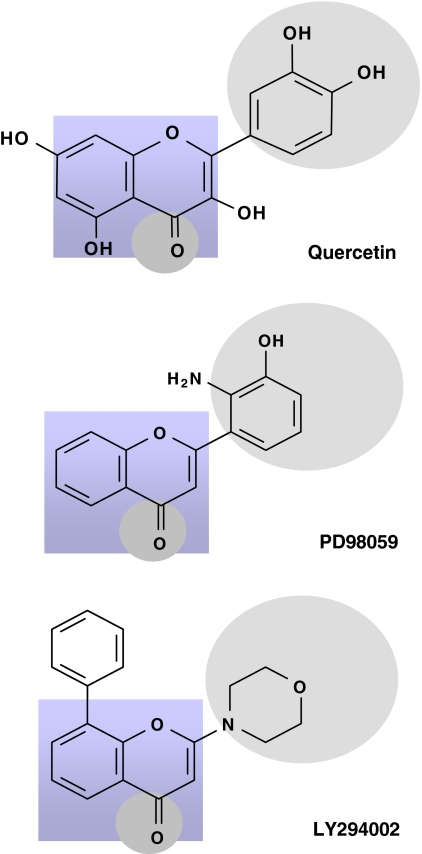
The ERK pathway is activated when Ras recruits c-Raf (a MAPKKK) to the membrane, resulting in its activation. Activated Raf then phosphorylates and activates MEK1/2 (MAPKK), which directly activates ERK through dual phosphorylation on threonine202 and tyrosine204 residues within the tripeptide motif TEY (Fig. 3). The ERK pathway is very responsive to growth factors and signals from certain G protein-coupled receptors and protein kinase C and two major isoforms of ERK, p44 (ERK1) and p42 (ERK2) have been identified [21]. Although most investigations have centred on the potential of flavonoids to modulate the phosphorylation state of ERK1/2 [74, 108, 148, 149, 159], it is highly likely that their actions on this MAPK isoform result from effects on upstream kinases, such as MEK1 and MEK2, and potentially membrane receptors [148]. This appears likely as flavonoids have close structural homology to specific inhibitors of ERK signalling, such as PD98059 (2′-amino-3′-methoxyflavone) (Fig. 6). PD98059 is a flavone that has been shown to act in vivo as a highly selective non-competitive inhibitor of MEK1 activation and the MAP kinase cascade [9, 52, 98, 130]. PD98059 acts via its ability to bind to the inactive forms of MEK so preventing its activation by upstream activators such as c-Raf [9] (Fig. 3). This raises the possibility that flavonoids, and their metabolites, may also act on this pathway in a similar manner. In support of this, the flavonol quercetin, and to a lesser extent its O-methylated metabolites have been shown to induce neuronal apoptosis via a mechanism involving the inhibition of ERK, rather than by induction of pro-apoptotic signalling through JNK [159]. The potent inhibition of ERK activation, and indeed Akt/PKB phosphorylation, was accompanied by activation of BAD and a subsequent strong activation of caspase-3.
Fig. 6.
The structure of MEK inhibitor PD98059 and the PI3K inhibitors, LY294002, have close structural homology to that of flavonoids. LY294002 and quercetin both fit into the ATP binding pocket of the PI3K, inhibiting its activity. It appears that the number and substitution of hydroxyl groups on the flavonoid B-ring and the degree of un-saturation of the C2–C3 bond are important determinants of their activity. Such inhibitory actions have been proposed as potential mechanisms by which flavonoids act to modulate neuronal function
There is increasing evidence that neuroinflammatory processes contribute to the progressive neuronal damage observed in neurodegenerative disorders such as AD [128] and PD [90, 177], and also with neuronal injury associated with stroke [10]. Central to neuroinflammation is the generation of nitric oxide (NO•) via increases in the expression of the inducible isoform of nitric oxide synthase (iNOS) in glial cells. Crucially the transcriptional regulation of iNOS in activated glial cells is dependent on signalling through the MAPK pathway, specifically through activation of ERK1/2 [16, 55, 112]. The MEK inhibitor PD98059 has been shown to effectively block iNOS expression and generation of NO• [16], suggesting that flavonoids may also be capable of exerting anti-inflammatory actions via inhibitory actions on MEK1 within the ERK signalling pathway. Thus far, studies have indicated that flavanols [72, 105], flavones [34, 87, 99, 151, 186], and flavonols [35] are all capable of inhibiting the release of NO• by activated microglia via the down-regulation of iNOS gene expression. However, it is not known if such effects are mediated by changes in signalling through ERK, or any other MAPK. Whether inhibition of MAPK signalling by flavonoids plays a role in the observed anti-neuroinflammatory effects requires further investigation.
Certain flavonoids have also been observed to exert a stimulatory effect on ERK1/2. For example, the flavan-3-ol, (−)-epicatechin, and one of its metabolites, 3′-O−methyl-(−)-epicatechin, have been shown to stimulate phosphorylation of ERK1/2 and the downstream transcription factor CREB at physiologically relevant concentrations [147]. Interestingly, this activation of the ERK pathway was no longer apparent at higher concentrations suggesting that effects on this pathway are concentration specific. Furthermore, stimulation of the ERK1/2 and CREB was not observed with (−)-epicatechin-5-O-β-d-glucuronide suggesting that effects on the ERK pathway may be dependent on cell or membrane permeability, as has been previously reported [160]. In support of these observations, the protective action of another flavanol, EGCG, against 6-hydroxy dopamine toxicity and serum deprivation has been shown to involve the restoration of both protein kinase C and ERK1/2 activities [104, 137].
One explanation for the concentration-specific regulation of the ERK pathway, and indeed other MAP kinase cascades (JNK and p38), may be related to the ability of flavonoids to exert high affinity receptor agonist-like actions at low concentrations and direct enzyme inhibition at higher concentrations [7, 179], or by inducing receptor desensitization. The identity of the primary flavonoid interacting sites in neurons is unknown and could be either at the cell surface or intracellular, although the ERK and PI3K dependence to CREB phosphorylation is very similar to ionotropic receptor signalling [133]. Receptors reported to act as flavonoid-binding sites, that are present in cortical neurons, are adenosine [79] and GABAA receptors [4, 80]. However, a specific plasma membrane binding site for polyphenols has recently been described in rat brain [69]. In addition, monomeric and dimeric flavanols show nanomolar affinity and efficacy at testosterone receptors [127] and resveratrol rapidly activates ERK signalling through alpha and beta oestrogen receptors [91]. Collectively, this raises the possibility that flavonoids may act on the ERK pathway via acting through steroid-like receptors in neurons to modulate ERK and CREB-mediated gene expression.
In addition to a receptor-mediated mechanism, it is equally plausibly that changes in ERK activation and related transcription factors (i.e. CREB) may result from flavonoid-induced modulation of phosphatase activity. Phosphatases act in opposition to kinases by de-phosphorylating specific kinases and in the process either activate or de-activate them. Consequently, phosphatases are integral to many signalling pathways. Because ERK and other MAPK require both Thr and Tyr phosphorylation for full activity, dual specificity phosphatases (DSPs) that de-phosphorylate both sites are uniquely positioned to regulate MAPK signal transduction cascades. At least nine DSPs, also termed MAPK phosphatases (MKPs), have been identified in mammalian cells [25]. DSPs frequently associated with ERK inactivation include MKP3, MKP4, and phosphatase of activated cells 1 (PAC1), although MKP3 (also termed PYST1) is probably the best studied and the most specific for ERK1/2 versus other MAPK [89]. The finding that multiple phosphatases inactivate the ERK pathway suggests that the duration and extent of ERK activation is controlled by a balance of the activities of upstream MAPKK, such as MEK1, and phosphatases, such MKP3. Although there has been intense interest in the ability of flavonoids to modulate kinases, thus far there is no indication that they may affect signalling pathways via a modulation of phosphatase activity. If flavonoids are capable for reacting with phosphatases, such as MKP3, then this is likely to have a dramatic effect on the activation states of important kinases like ERK1/2. Future investigations in this area should consider the potential of flavonoids to inhibit, or activate phosphatases, the concentration-dependency of these effects and the mechanism by which they do so.
Stress-activated protein kinases: c-Jun-N-terminal kinase (JNK) and p38
c-Jun-N-terminal kinase (JNK) is regulated by a variety of MAPKKK’s, including MEKK1/4 and apoptosis signal-related kinase (ASK1) [26, 76], which is further regulated by GTPases and Rac1 [120, 121] (Fig. 4). Unlike the ERK pathway, the JNK cascade is strongly activated by stress signals such as UV and γ-radiation, oxidative stress and inflammatory cytokines [45, 75, 97, 103, 184]. JNK itself is activated by MKK4/7, a MAPKK, via dual phosphorylation within the Thr138-Pro-Tyr-185 motif (pTPpY) in its catalytic core. There is very strong evidence linking the activation of JNK to neuronal injury in response to a wide array of pro-apoptotic stimuli in both developmental and degenerative death signalling [45, 46, 118]. In the context of oxidative insults in neurons, JNK has been found to be activated by dopamine [110], by 4-HNE [24, 132, 155] and through reduced expression of superoxide dismutase (SOD) 1 [113]. In addition, the activation of JNK pathway and the death of specific neuronal populations is crucial during early brain development [103]. As with the other MAP kinases, the core signalling unit is composed of a MAPKKK, typically MEKK1-4, which phosphorylate and activate MKK4-7, which then phosphorylate and activate the JNK [45, 75]. Another MAPKKK, apoptosis signal-regulating kinase 1 (ASK1), also plays an essential role in stress-induced apoptosis [76, 182]. ASK1 can be activated in response to a variety of stress-related stimuli, including oxidative stress and activates MKK4, which in turn activates JNK (Fig. 4) and indeed p38 (Fig. 5) [115]. Overexpression of ASK1 has been shown to induce the activation of both JNK and p38 and lead to apoptosis via signals involving the mitochondrial cell death pathway [76, 103].
The flavanols, epicatechin and 3′-O-methyl-epicatechin have been shown to protect neurons against oxidative damage via a mechanism involving the suppression of JNK, and downstream partners, c-jun and pro-caspase-3 [149, 161]. In support of these observations, the flavone, baicalein, has been shown to significantly inhibit 6-OHDA-induced JNK activation and neuronal cell death and quercetin may suppress JNK activity and apoptosis induced by hydrogen peroxide [78, 181], 4-hydroxy-2-nonenal [173] and tumour necrosis factor-alpha (TNF-alpha) [92]. There are a number of potential sites where flavonoids may interact with the JNK pathway. For instance, flavonoid-mediated inhibition of oxidative stress-induced apoptosis may occur by preventing the activation of JNK by influencing one of the many upstream MAPKKK activating proteins that transduce signals to JNK (Fig. 4). Their ability to inhibit JNK activation may proceed via flavonoid-induced modulation of the ASK1 phosphorylation state, and its association with 14-3-3 protein, which is essential for suppression of cellular apoptosis [201]. Oxidative stress in known to regulate the activity of this MAPKKK by causing the de-phosphorylation of ASK1 at Ser967 and its phosphorylation at Thr845 in the activation loop, both of which are correlated with ASK1 activity and ASK1-dependent apoptosis [62, 171]. Flavonoids may modulate ASK1 activity by affecting these phosphorylation sites, or by influencing PI3K/Akt signalling, which is known to induce phosphorylation of ASK1 at Ser83, an event which attenuates ASK1 activity and promotes cell survival [86].
Another potential mechanism by which flavonoids act is through the ability to preserve Ca2+ homeostasis, thereby preventing Ca2+-dependent activation of JNK [45, 149]. Calcium dependent MAPK signalling has been suggested to play a role in glutamate receptor-mediated neuronal stress [133]. The influx of Ca2+ into the cytosol from the extracellular space or from intracellular stores following stress stimuli may activate Ca2+/calmodulin kinases, which in turn can stimulate the activation of all three MAPK, especially JNK [190]. Alternatively, flavonoids may exert direct interactions with upstream signalling components required for the recruitment and activation of JNK in neurons. Studies have indicated that some flavonoids may be capable of exerting neuroprotective actions through an attenuation of the pro-apoptotic signalling cascade lying downstream of JNK. Phosphorylation of the AP-1 protein c-Jun on Ser-63 and Ser-73 by JNK causes increased transcriptional activity [134], which has been linked to stress-induced apoptosis. Activated c-Jun is known to regulate the expression of pro-apoptotic genes that may activate Bax, leading to the release of cytochrome c from mitochondria, and culminating in a stimulation of caspases 9 and 3 [198]. Investigation has indicated that oxidative-induced activation of caspase-3 in neurons is blocked by flavonoids, providing compelling evidence in support of a potent anti-apoptotic action of flavonoids in these cells [149, 150, 160].
The p38 mitogen-activated protein kinase also plays an important role in the signal transduction of extracellular signals into the nucleus [125, 143] (Fig. 5). Similar to the JNK pathway, p38 MAP kinase is activated by a variety of cellular stresses including osmotic shock, pro-inflammatory inflammatory cytokines, lipopolysacharides, UV light and growth factors [68, 100, 125, 135, 144]. The p38 pathway is regulated by the MKKs, MKK3 and MKK6, which in turn, are regulated by the upstream MAPKKK, MEK1/4 and ASK [125] (Fig. 5). The activation of p38 has been shown to lead to the phosphorylation of the transcription factors ATF-2 [135], Max [199] and MEF2 [191, 202]. Such targets, in particular ATF-2 and MEF2 are known to regulate various forms of cellular stress, including genotoxic agents, inflammatory cytokines and UV irradiation.
There are very few investigations that have addressed the ability of flavonoids to modulate neuronal signalling through the p38 pathway. However, there are some studies which suggest that flavonoids may interact within the p38 pathway in other cells types, for example in human mammary epithelial cells [59], thus hinting that they may be able to induce neuronal effects via this pathway. The challenge now is to determine the precise site(s) of action of flavonoids within the p38 cascade and the sequence of events that allow them to regulate stress-induced neuronal death in the central nervous system. One such potential site of action may be specific redox-sensitive motifs, notably cysteine residues, similar to those reported for JNK [131]. JNK redox regulation has been proposed to proceed through its binding to redox sensitive proteins such as glutathione-S-transferase (GST) [5, 6, 123]. It has been shown that under unstressed conditions, JNK is associated with GST resulting in the inhibition of JNK activity, but that JNK dissociates from GST following UV or oxidative stress [5, 192]. Flavonoids also may act to inhibit JNK activity, and other MAPK members, via the nucleophilic addition of flavonoid o-quinones, formed from the intracellular oxidation of flavonoids [156, 158], to these cysteine residues.
PI3 kinase signalling pathway
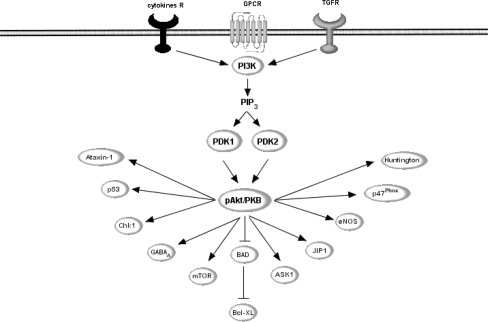
In addition to MAPK pathway, flavonoids have been shown to modulate signalling through the serine/threonine kinase, Akt/PKB, one of the main downstream effectors of PI3K, a pivotal kinase in neuronal survival [37, 40, 85, 119] (Fig. 7). PI3K is a heterodimer of a catalytic subunit (p110) and a regulatory subunit (p85) [27, 31, 82] and it is thought that binding between PI3K and phospholipids is due to the ability of the regulatory subunit to interact with both the catalytic subunit and receptor tyrosine kinases. Active PI3K catalyzes the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) by phosphorylating phosphatidylinositol (PI), phosphatidylinositol-4-phosphate (PIP) and phosphatidylinositol-4,5-bisphosphate (PIP2). PIP3 may then activate phosphoinositide-dependent protein kinase 1 (PDK1), which plays a central role in many signal transduction pathways [31, 153], activating Akt and the PKC isoenzymes p70 S6 kinase and RSK [126]. Through its effects on these kinases, PDK1 is involved in the regulation of a wide variety of processes, including cell growth, cell proliferation, differentiation, cell cycle entry, cell migration and apoptosis [31].
Fig. 7.
Potential points of action of flavonoids within PI3K/Akt signalling pathway. Active PI3K catalyzes the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) which activates phosphoinositide-dependent protein kinase 1 (PDK1). PDK1 plays a central role in many signal transduction pathways, activating Akt and the PKC isoenzymes p70 S6 kinase and RSK. Through its effects on these kinases, PI3K is involved in the regulation of a wide variety of processes, including cell growth, cell proliferation, differentiation, cell cycle entry, cell migration and apoptosis. Flavonoids have been proposed to act on this pathway via direct modulation of PI3K activity via binding to its ATP binding pocket, in a similar manner to that of LY294002. Alternatively, they may act to modulate the activity of the tumour suppressor, PTEN
One of the most important targets of PI3K and PDK1 is Akt, (also known as Protein Kinase B), as this kinase plays a critical role in controlling cellular survival and apoptosis [22, 57, 58]. Akt promotes cell survival by inhibiting apoptosis through its ability to phosphorylate and inactivate several important targets, including Bad [30], Forkhead transcription factors [20, 23] and caspase-9 [22] (Fig. 6). Indeed, activation of Akt/PKB in neurons has been shown to lead to an inhibition of proteins central to neuronal death machinery, such as the pro-apoptotic Bcl-2 family member, BAD [200], and members of the caspase family [19, 85] that specifically cleave poly(ADP-ribose) polymerase [37, 85], thus promoting cell survival. Akt is activated by phospholipid binding and activation loop phosphorylation at Thr308 by PDK1 [8] and by phosphorylation within the carboxy terminus at Ser473 and the activation of Akt is a pro-survival event in many cell types due to its ability to inactivate BAD via phosphorylation at Ser136 [43, 49].
There is powerful evidence that flavonoids inhibit PI3K via direct interactions with its ATP binding site. Indeed, a number of studies have demonstrated that the structure of flavonoids determines whether or not they act as potent inhibitors of PI3K [7, 54]. One of the most selective PI3K inhibitors available, LY294002 (Fig. 5), was modelled on the structure of quercetin [116, 178]. LY294002 and quercetin fit into the ATP binding pocket of the enzyme although with surprisingly different orientations [179]. It appears that the number and substitution of hydroxyl groups on the B-ring and the degree of un-saturation of the C2–C3 bond are important determinants of this particular bioactivity. Interestingly in this regard quercetin and some of its in vivo metabolites inhibit pro-survival Akt/PKB signalling pathways [159] by a mechanism of action consistent with the flavonoids binding to and inhibiting PI3K activity. Prior to inducing measurable losses of neuronal viability, quercetin stimulates a strong inhibition of basal Akt phosphorylation at both the regulatory serine473 and catalytic threonine308 sites, rendering it inactive. The inhibition of Akt/PKB phosphorylation in this way may reflect potential inhibition of its upstream partner PI3K, as has previously been described [116]. If Akt/PKB inhibition is sustained, which has been reported to occur during neuronal exposure to quercetin, this leads to extensive caspase-3 activation and subsequent caspase-dependent cleavage of Akt/PKB, an event that effectively turns off the major survival signal and results in the acceleration of apoptotic death [159]. However, at lower concentrations, quercetin has also been shown to trigger CREB activation in neurons indicating that exposure concentration is pivotal in determining either pro-apoptotic or anti-apoptotic effects [159]. Indeed, low concentrations of quercetin, may activate the MAPK pathway (ERK2, JNK1 and p38) leading to expression of survival genes (c-Fos, c-Jun) and defensive genes (Phase II detoxifying enzymes; glutathione-S-transferase, quinone reductase) resulting in survival and protective mechanisms (homeostasis response), whereas high concentrations stimulate pro-apoptotic pathways and ultimate caspase activation [93].
Another potential mechanism by which flavonoids may modulate the PI3 kinase/Akt signalling pathway is by their ability to modulate the expression or activity of PTEN (phosphatase and tensin homologue deleted on chromosome ten), also referred to as MMAC (mutated in multiple advanced cancers) phosphatase [29, 44, 66]. PTEN is a tumour suppressor implicated in a wide variety of human cancers [28] and the main substrates of PTEN are inositol phospholipids generated by the activation of the PI3K [124]. PTEN acts a major negative regulator of the PI3K/Akt signalling pathway [28, 187] and thus a modulation of its expression or activation by flavonoids will have a profound effect of cellular function. For example, if flavonoids are capable of inhibiting PTEN in cancer cells this may lead to an increase in cancer cell proliferation and tumour growth. On the other hand, its activation in post-mitotic cells, such as neurons, may have a positive effect by increasing Akt and CREB activity leading to a promotion of neuronal survival and synaptic plasticity (Fig. 2).
Conclusions
Emerging evidence suggests that dietary phytochemicals, in particular flavonoids, may exert beneficial effects in the CNS by protecting neurons against stress induced injury, by suppressing the activation of microglia and astrocytes, which mediate neuroinflammation, and by promoting synaptic plasticity, memory and cognitive function. Evidence supports the localization of flavonoids within the brain, thus these phytochemicals may be regarded as a potential neuroprotective, neuromodulatory or anti-neuroinflammatory agents. It appears highly likely that such beneficial properties are mediated by their abilities to interact with both protein and lipid kinase signalling cascades, rather then via their potential to act as antioxidants. The concentrations of flavonoids encountered in vivo are sufficiently high to exert pharmacological activity at receptors, kinases and transcription factors. However, precise sites of action are presently unknown. It is likely that their activity depends on their ability to: (1) bind to ATP sites on enzymes and receptors; (2) modulate the activity of kinases directly, i.e. MAPKKK, MAPKK or MAPK; (3) affect the function of important phosphatases, which act in opposition to kinases; (4) preserve Ca2+ homeostasis, thereby preventing Ca2+-dependent activation of kinases in neurons; and (5) modulate signalling cascades lying downstream of kinases, i.e. transcription factor activation and binding to promoter sequences.
Another unknown factor relates to the precise cellular site of action of flavonoids. For example, does flavonoid action require cellular uptake or are they capable of mediating effects via extracellular receptor binding. Presently, there is no certainty either way, although flavonoid glucuronides, which are unable to enter cells to any significant degree, do not express cellular effects. This may suggest a requirement for cytosolic localisation, although it could equally signify that the conjugation of flavonoids with glucuronide or sulphate moieties blocks receptor binding and therefore their cellular activity. It appears likely that the inhibition of Akt is almost certainly mediated via actions at PI3K, thus requiring cellular uptake. However, actions at ERK1/2 could result from either flavonoid modulation of upstream regulatory kinases or by binding directly to receptors. The challenge now is to determine the precise site(s) of action of flavonoids within the signalling pathways and the sequence of events that allow them to regulate neuronal function in the central nervous system.
Ultimately actions within these neuronal signalling cascades may be beneficial or negative in the context of the brain. For example, whilst they may be positive in the treatment of proliferative diseases, they could be detrimental to the nervous system, at least at high concentrations, where these same pathways act to control neuronal survival and synaptic plasticity. Thus, flavonoid interactions with intracellular signalling pathways could have unpredictable outcomes and will be dependent on the cell type (i.e. neurons, astrocytes, microglia, oligodendrocytes), the disease studied and the stimulus applied. In summary, it is evident that flavonoids are potent bioactive molecules and a clear understanding of their mechanisms of action as modulators of cell signalling will be crucial in the evaluation of their potential to act as inhibitors of neurodegeneration or as modulators of brain function.
Acknowledgments
Dr. Jeremy Spencer is sponsored by the Biotechnology and Biological Sciences Research Council (BB/C518222/1) and the Medical Research Council (G0400278/NI02).
Abbreviations
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated protein kinase
- JNK
c-Jun N-terminal kinase
- PI3K
Phosphatidylinositol-3 kinase
- PKB
Protein kinase B
- PKC
Protein kinase C
- ASK1
Apoptosis signal-regulating kinase 1
- STAT-1
Signal transducer and activator of transcription-1
- AP-1
Activated protein-1
- CREB
cAMP response element-binding protein
- ATF-1/2
Activating transcription factor 1/2
- MSK1
Mitogen- and stress-activated protein kinase 1
- MTOR
Mammalian target of rapamycin
- p47phox
NADPH oxidase (p47 cytoplasmic element; p90RSK (RSK): 90 kDa ribosomal S6 kinase
- MEF-2
Myocyte enhancer factor 2
- DSP
Dual specificity phosphatase
- PTEN
Phosphatase and tensin homologue deleted on chromosome ten
- LPS
Lipopolysacharide
- IL-1β
Interleukin-1β
- INOS
Inducible nitric oxide synthase
- ENOS
Endothelial nitric oxide synthase
- IFN-γ
Interferon gamma
- LDL
Low-density lipoprotein
- NO•
Nitric oxide
- TNF-α
Tumor necrosis factor-alpha
- BBB
Blood brain barrier
- CNS
Central nervous system
References
- 1.Aasmundstad TA, Morland J, Paulsen RE (1995) Distribution of morphine 6-glucuronide and morphine across the blood–brain barrier in awake, freely moving rats investigated by in vivo microdialysis sampling. J Pharmacol Exp Ther 275:435–441 [PubMed]
- 2.Abbott NJ (2002) Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat 200:629–638 [DOI] [PMC free article] [PubMed]
- 3.Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA (2002) Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 33:1693–1702 [DOI] [PubMed]
- 4.Adachi N, Tomonaga S, Tachibana T, Denbow DM, Furuse M (2006) (−)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur J Pharmacol 531:171–175 [DOI] [PubMed]
- 5.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z (1999a) Regulation of JNK signaling by GSTp. EMBO J 18:1321–1334 [DOI] [PMC free article] [PubMed]
- 6.Adler V, Yin Z, Tew KD, Ronai Z (1999b) Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18:6104–6111 [DOI] [PubMed]
- 7.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B (1997) Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol 53:1649–1657 [DOI] [PubMed]
- 8.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551 [PMC free article] [PubMed]
- 9.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270:27489–27494 [DOI] [PubMed]
- 10.Allan SM, Rothwell NJ (2003) Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci 358:1669–1677 [DOI] [PMC free article] [PubMed]
- 11.Anderson CN, Tolkovsky AM (1999) A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19:664–673 [DOI] [PMC free article] [PubMed]
- 12.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA (2005) Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci 8:111–120 [DOI] [PubMed]
- 13.Barzilai A, Rahamimoff H (1983) Inhibition of Ca2+-transport ATPase from synaptosomal vesicles by flavonoids. Biochim Biophys Acta 730:245–254 [DOI] [PubMed]
- 14.Bastianetto S, Zheng WH, Quirion R (2000) The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. J Neurochem 74:2268–2277 [DOI] [PubMed]
- 15.Behrens A, Sibilia M, Wagner EF (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 21:326–329 [DOI] [PubMed]
- 16.Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641 [DOI] [PMC free article] [PubMed]
- 17.Blanc A, Pandey NR, Srivastava AK (2003) Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease (review). Int J Mol Med 11:229–234 [PubMed]
- 18.Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Andersen A, Jakob F, Kohrle J (1996) Selected novel flavones inhibit the DNA binding or the DNA religation step of eukaryotic topoisomerase I. J Biol Chem 271:2262–2270 [DOI] [PubMed]
- 19.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362 [DOI] [PubMed]
- 20.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed]
- 21.Burgering BM, Bos JL (1995) Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci 20:18–22 [DOI] [PubMed]
- 22.Burgering BM, Coffer PJ (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599–602 [DOI] [PubMed]
- 23.Burgering BM, Kops GJ (2002) Cell cycle and death control: long live Forkheads. Trends Biochem Sci 27:352–360 [DOI] [PubMed]
- 24.Camandola S, Poli G, Mattson MP (2000) The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem 74:159–168 [DOI] [PubMed]
- 25.Camps M, Nichols A, Arkinstall S (2000) Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14:6–16 [PubMed]
- 26.Cano E, Mahadevan LC (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20:117–122 [DOI] [PubMed]
- 27.Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657 [DOI] [PubMed]
- 28.Cantley LC, Neel BG (1999) New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96:4240–4245 [DOI] [PMC free article] [PubMed]
- 29.Cao F, Jin TY, Zhou YF (2006) Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene. Biomed Environ Sci 19:35–41 [PubMed]
- 30.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321 [DOI] [PubMed]
- 31.Carpenter CL, Cantley LC (1990) Phosphoinositide kinases. Biochemistry 29:11147–11156 [DOI] [PubMed]
- 32.Castagne V, Clarke PG (1999) Inhibitors of mitogen-activated protein kinases protect axotomized developing neurons. Brain Res 842:215–219 [DOI] [PubMed]
- 33.Castagne V, Gautschi M, Lefevre K, Posada A, Clarke PG (1999) Relationships between neuronal death and the cellular redox status. Focus on the developing nervous system. Prog Neurobiol 59:397–423 [DOI] [PubMed]
- 34.Chen CJ, Raung SL, Liao SL, Chen SY (2004) Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem Pharmacol 67:957–965 [DOI] [PubMed]
- 35.Chen JC, Ho FM, Pei-Dawn LC, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen TW, Lin WW (2005) Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 521:9–20 [DOI] [PubMed]
- 36.Cobb MH, Goldsmith EJ (1995) How MAP kinases are regulated. J Biol Chem 270:14843–14846 [DOI] [PubMed]
- 37.Coffer PJ, Jin J, Woodgett JR (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335(Pt 1):1–13 [DOI] [PMC free article] [PubMed]
- 38.Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A (1998) Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA 95:9831–9836 [DOI] [PMC free article] [PubMed]
- 39.Crossthwaite AJ, Hasan S, Williams RJ (2002) Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca(2+) and PI3-kinase. J Neurochem 80:24–35 [DOI] [PubMed]
- 40.Crowder RJ, Freeman RS (1998) Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 18:2933–2943 [DOI] [PMC free article] [PubMed]
- 41.da Silva EL, Piskula MK, Yamamoto N, Moon JH, Terao J (1998) Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Lett 430:405–408 [DOI] [PubMed]
- 42.Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT (2001) Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 12:3871–3875 [DOI] [PubMed]
- 43.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241 [DOI] [PubMed]
- 44.Dave B, Eason RR, Till SR, Geng Y, Velarde MC, Badger TM, Simmen RC (2005) The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis 26:1793–1803 [DOI] [PubMed]
- 45.Davis RJ (1999) Signal transduction by the c-Jun N-terminal kinase. Biochem Soc Symp 64:1–12 [DOI] [PubMed]
- 46.Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252 [DOI] [PubMed]
- 47.Day AJ, Williamson G (2003) Absorption of quercetin glycosides. In: Rice-Evans C, Packer L (eds) Flavonoids in health and disease. Marcel Dekker, New York, pp 391–412
- 48.Dekermendjian K, Kahnberg P, Witt MR, Sterner O, Nielsen M, Liljefors T (1999) Structure–activity relationships and molecular modeling analysis of flavonoids binding to the benzodiazepine site of the rat brain GABA(A) receptor complex. J Med Chem 42:4343–4350 [DOI] [PubMed]
- 49.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689 [DOI] [PubMed]
- 50.Di Pietro A, Godinot C, Bouillant ML, Gautheron DC (1975) Pig heart mitochondrial ATPase: properties of purified and membrane-bound enzyme. Effects of flavonoids. Biochimie 57:959–967 [DOI] [PubMed]
- 51.Donovan JL, Waterhouse AL (2003) Bioavailability of flavanol monomers. In: Rice-Evans C, Packer L (eds). Flavonoids in health and disease. Marcel Dekker, New York, pp 413–440
- 52.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR (1995) A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92:7686–7689 [DOI] [PMC free article] [PubMed]
- 53.El Mohsen MA, Marks J, Kuhnle G, Moore K, Debnam E, Kaila SS, Rice-Evans C, Spencer JP (2006) Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br J Nutr 95:51–58 [DOI] [PubMed]
- 54.Ferriola PC, Cody V, Middleton E Jr (1989) Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem Pharmacol 38:1617–1624 [DOI] [PubMed]
- 55.Fiebich BL, Lieb K, Engels S, Heinrich M (2002) Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol 132:18–24 [DOI] [PubMed]
- 56.Fischer PM, Lane DP (2000) Inhibitors of cyclin-dependent kinases as anti-cancer therapeutics. Curr Med Chem 7:1213–1245 [DOI] [PubMed]
- 57.Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437 [DOI] [PubMed]
- 58.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727–736 [DOI] [PubMed]
- 59.Frey RS, Singletary KW (2003) Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J Nutr 133:226–231 [DOI] [PubMed]
- 60.Galli RL, Shukitt-Hale B, Youdim KA, Joseph JA (2002) Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Ann N Y Acad Sci 959:128–132 [DOI] [PubMed]
- 61.Gamet-Payrastre L, Manenti S, Gratacap MP, Tulliez J, Chap H, Payrastre B (1999) Flavonoids and the inhibition of PKC and PI 3-kinase. Gen Pharmacol 32:279–286 [DOI] [PubMed]
- 62.Goldman EH, Chen L, Fu H (2004) Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem 279:10442–10449 [DOI] [PubMed]
- 63.Goldsmith EJ, Cobb MH (1994) Protein kinases. Curr Opin Struct Biol 4:833–840 [DOI] [PubMed]
- 64.Goyal L (2001) Cell death inhibition: keeping caspases in check. Cell 104:805–808 [DOI] [PubMed]
- 65.Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312 [DOI] [PubMed]
- 66.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M (2006) The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res 26:1177–1181 [PubMed]
- 67.Halliwell B, Zhao K, Whiteman M (2000) The gastrointestinal tract: a major site of antioxidant action?. Free Radic Res 33:819–830 [DOI] [PubMed]
- 68.Han J, Lee JD, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808–811 [DOI] [PubMed]
- 69.Han YS, Bastianetto S, Dumont Y, Quirion R (2006) Specific plasma membrane binding sites for polyphenols, including resveratrol, in the rat brain. J Pharmacol Exp Ther 318:238–245 [DOI] [PubMed]
- 70.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O (2006) Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr 136:1043–1047 [DOI] [PubMed]
- 71.Herdegen T, Skene P, Bahr M (1997) The c-Jun transcription factor-bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci 20:227–231 [DOI] [PubMed]
- 72.Huang Q, Wu LJ, Tashiro S, Gao HY, Onodera S, Ikejima T (2005) (+)-Catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced DNA damage and cell cycle arrest. J Pharmacol Sci 98:16–24 [DOI] [PubMed]
- 73.Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E Jr, Lee MT (1999) Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 128:999–1010 [DOI] [PMC free article] [PubMed]
- 74.Hung SP, Hsu JR, Lo CP, Huang HJ, Wang JP, Chen ST (2005) Genistein-induced neuronal differentiation is associated with activation of extracellular signal-regulated kinases and upregulation of p21 and N-cadherin. J Cell Biochem 96:1061–1070 [DOI] [PubMed]
- 75.Ichijo H (1999) From receptors to stress-activated MAP kinases. Oncogene 18:6087–6093 [DOI] [PubMed]
- 76.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90–94 [DOI] [PubMed]
- 77.Inanami O, Watanabe Y, Syuto B, Nakano M, Tsuji M, Kuwabara M (1998) Oral administration of (−)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Radic Res 29:359–365 [DOI] [PubMed]
- 78.Ishikawa Y, Kitamura M (2000) Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 58:1078–1087 [DOI] [PubMed]
- 79.Jacobson KA, Moro S, Manthey JA, West PL, Ji XD (2002) Interactions of flavones and other phytochemicals with adenosine receptors. Adv Exp Med Biol 505:163–171 [DOI] [PMC free article] [PubMed]
- 80.Johnston GA (2005) GABA(A) receptor channel pharmacology. Curr Pharm Des 11:1867–1885 [DOI] [PubMed]
- 81.Kantengwa S, Polla BS (1991) Flavonoids, but not protein kinase C inhibitors, prevent stress protein synthesis during erythrophagocytosis. Biochem Biophys Res Commun 180:308–314 [DOI] [PubMed]
- 82.Kapeller R, Cantley LC (1994) Phosphatidylinositol 3-kinase. Bioessays 16:565–576 [DOI] [PubMed]
- 83.Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10:381–391 [DOI] [PubMed]
- 84.Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486 [DOI] [PubMed]
- 85.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713 [DOI] [PubMed]
- 86.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV (2001a) Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 21:893–901 [DOI] [PMC free article] [PubMed]
- 87.Kim H, Kim YS, Kim SY, Suk K (2001b) The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci Lett 309:67–71 [DOI] [PubMed]
- 88.Kim JS, He L, Lemasters JJ (2003a) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304:463–470 [DOI] [PubMed]
- 89.Kim Y, Rice AE, Denu JM (2003b) Intramolecular dephosphorylation of ERK by MKP3. Biochemistry 42:15197–15207 [DOI] [PubMed]
- 90.Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med 38:333–347 [DOI] [PubMed]
- 91.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS (2005) Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 280:7460–7468 [DOI] [PubMed]
- 92.Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L (1999) Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol 277:C403–C411 [DOI] [PubMed]
- 93.Kong AN, Yu R, Chen C, Mandlekar S, Primiano T (2000) Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res 23:1–16 [DOI] [PubMed]
- 94.Kroemer HK, Klotz U (1992) Glucuronidation of drugs. A re-evaluation of the pharmacological significance of the conjugates and modulating factors. Clin Pharmacokinet 23:292–310 [DOI] [PubMed]
- 95.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I (2006) Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr 83:355–361 [DOI] [PubMed]
- 96.Kwon YW, Masutani H, Nakamura H, Ishii Y, Yodoi J (2003) Redox regulation of cell growth and cell death. Biol Chem 384:991–996 [DOI] [PubMed]
- 97.Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–869 [DOI] [PubMed]
- 98.Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR (1995) Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem 270:20801–20807 [DOI] [PubMed]
- 99.Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K (2003) Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 17:1943–1944 [DOI] [PubMed]
- 100.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–746 [DOI] [PubMed]
- 101.Lee SF, Lin JK (1997) Inhibitory effects of phytopolyphenols on TPA-induced transformation, PKC activation, and c-jun expression in mouse fibroblast cells. Nutr Cancer 28:177–183 [DOI] [PubMed]
- 102.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA (2002) Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal 4:769–781 [DOI] [PubMed]
- 103.Leppa S, Bohmann D (1999) Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18:6158–6162 [DOI] [PubMed]
- 104.Levites Y, Amit T, Youdim MB, Mandel S (2002) Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 277:30574–30580 [DOI] [PubMed]
- 105.Li R, Huang YG, Fang D, Le WD (2004) (−)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res 78:723–731 [DOI] [PubMed]
- 106.Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW (2001) A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 31:841–851 [DOI] [PubMed]
- 107.Lin JH, Yamazaki M (2003) Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet 42:59–98 [DOI] [PubMed]
- 108.Llorens F, Garcia L, Itarte E, Gomez N (2002) Apigenin and LY294002 prolong EGF-stimulated ERK1/2 activation in PC12 cells but are unable to induce full differentiation. FEBS Lett 510:149–153 [DOI] [PubMed]
- 109.Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P (2002) Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci USA 99:12197–12202 [DOI] [PMC free article] [PubMed]
- 110.Luo Y, Umegaki H, Wang X, Abe R, Roth GS (1998) Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem 273:3756–3764 [DOI] [PubMed]
- 111.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747 [DOI] [PubMed]
- 112.Marcus JS, Karackattu SL, Fleegal MA, Sumners C (2003) Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia 41:152–160 [DOI] [PubMed]
- 113.Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM, Park DS, Yan CY, Troy CM, Greene LA (1999) CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem 73:1901–1912 [PubMed]
- 114.Marshall CJ (1994) Signal transduction. Hot lips and phosphorylation of protein kinases. Nature 367:686 [DOI] [PubMed]
- 115.Matsuzawa A, Ichijo H (2001) Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem (Tokyo) 130:1–8 [DOI] [PubMed]
- 116.Matter WF, Brown RF, Vlahos CJ (1992) The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun 186:624–631 [DOI] [PubMed]
- 117.Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, Paladini AC (1997) Overview-flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res 22:419–425 [DOI] [PubMed]
- 118.Mielke K, Herdegen T (2000) JNK and p38 stresskinases-degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol 61:45–60 [DOI] [PubMed]
- 119.Miller FD, Kaplan DR (2001) Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci 58:1045–1053 [DOI] [PMC free article] [PubMed]
- 120.Minden A, Lin A, Claret FX, Abo A, Karin M (1995) Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147–1157 [DOI] [PubMed]
- 121.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M (1994) Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719–1723 [DOI] [PubMed]
- 122.Miyake Y, Shimoi K, Kumazawa S, Yamamoto K, Kinae N, Osawa T (2000) Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treated rats. J Agric Food Chem 48:3217–3224 [DOI] [PubMed]
- 123.Monaco R, Friedman FK, Hyde MJ, Chen JM, Manolatus S, Adler V, Ronai Z, Koslosky W, Pincus MR (1999) Identification of a glutathione-S-transferase effector domain for inhibition of jun kinase, by molecular dynamics. J Protein Chem 18:859–866 [DOI] [PubMed]
- 124.Myers MP, Pass I, Batty IH, Van der KJ, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK (1998) The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 95:13513–13518 [DOI] [PMC free article] [PubMed]
- 125.Nebreda AR, Porras A (2000) p38 MAP kinases: beyond the stress response. Trends Biochem Sci 25:257–260 [DOI] [PubMed]
- 126.Neri LM, Borgatti P, Capitani S, Martelli AM (2002) The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta 1584:73–80 [DOI] [PubMed]
- 127.Nifli AP, Bosson-Kouame A, Papadopoulou N, Kogia C, Kampa M, Castagnino C, Stournaras C, Vercauteren J, Castanas E (2005) Monomeric and oligomeric flavanols are agonists of membrane androgen receptors. Exp Cell Res 309:329–339 [DOI] [PubMed]
- 128.Ong WY, Farooqui AA (2005) Iron, neuroinflammation, and Alzheimer’s disease. J Alzheimers Dis 8:183–200 [DOI] [PubMed]
- 129.Owuor ED, Kong AN (2002) Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol 64:765–770 [DOI] [PubMed]
- 130.Pang L, Sawada T, Decker SJ, Saltiel AR (1995) Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 270:13585–13588 [DOI] [PubMed]
- 131.Park HS, Park E, Kim MS, Ahn K, Kim IY, Choi EJ (2000) Selenite inhibits the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) through a thiol redox mechanism. J Biol Chem 275:2527–2531 [DOI] [PubMed]
- 132.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU (1998) HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest 102:1942–1950 [DOI] [PMC free article] [PubMed]
- 133.Perkinton MS, Sihra TS, Williams RJ (1999) Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J Neurosci 19:5861–5874 [DOI] [PMC free article] [PubMed]
- 134.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR (1991) Phosphorylation of c-jun mediated by MAP kinases. Nature 353:670–674 [DOI] [PubMed]
- 135.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270:7420–7426 [DOI] [PubMed]
- 136.Revuelta MP, Cantabrana B, Hidalgo A (1997) Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol 29:847–857 [DOI] [PubMed]
- 137.Reznichenko L, Amit T, Youdim MB, Mandel S (2005) Green tea polyphenol (−)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J Neurochem 93:1157–1167 [DOI] [PubMed]
- 138.Rice-Evans C (1995) Plant polyphenols: free radical scavengers or chain-breaking antioxidants? Biochem Soc Symp 61:103–116 [DOI] [PubMed]
- 139.Rice-Evans C (2001) Flavonoid antioxidants. Curr Med Chem 8:797–807 [DOI] [PubMed]
- 140.Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956 [DOI] [PubMed]
- 141.Rincon M, Flavell RA, Davis RA (2000) The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med 28:1328–1337 [DOI] [PubMed]
- 142.Rosenblat M, Belinky P, Vaya J, Levy R, Hayek T, Coleman R, Merchav S, Aviram M (1999) Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein. A possible role for protein kinase C. J Biol Chem 274:13790–13799 [DOI] [PubMed]
- 143.Rouse J, Cohen P, Trigon S, Morange M, onso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR (1994a) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027–1037 [DOI] [PubMed]
- 144.Rouse J, Cohen P, Trigon S, Morange M, onso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR (1994b) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027–1037 [DOI] [PubMed]
- 145.Saeki K, Kobayashi N, Inazawa Y, Zhang H, Nishitoh H, Ichijo H, Saeki K, Isemura M, Yuo A (2002) Oxidation-triggered c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase pathways for apoptosis in human leukaemic cells stimulated by epigallocatechin-3-gallate (EGCG): a distinct pathway from those of chemically induced and receptor-mediated apoptosis. Biochem J 368:705–720 [DOI] [PMC free article] [PubMed]
- 146.Scheline RR (1999) Metabolism of oxygen heterocyclic compounds, In: CRC Handbook of mammalian metabolism of plant compounds. CRC, Boca Ranton, pp 243–295
- 147.Schroeter H, Bahia P, Spencer JPE, Sheppard O, Rattray M, Rice-Evans C, Williams RJ (2007) (−)-epicatechin stimulates ERK-dependent cyclic AMP response element activity and upregulates GLUR2 in cortical neurons. J Neurochem 101:1596–1606 [DOI] [PubMed]
- 148.Schroeter H, Boyd C, Spencer JPE, Williams RJ, Cadenas E, Rice-Evans C (2002) MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging 23:861–880 [DOI] [PubMed]
- 149.Schroeter H, Spencer JP, Rice-Evans C, Williams RJ (2001) Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J 358:547–557 [DOI] [PMC free article] [PubMed]
- 150.Schroeter H, Williams RJ, Matin R, Iversen L, Rice-Evans CA (2000) Phenolic antioxidants attenuate neuronal cell death following uptake of oxidized low-density lipoprotein. Free Radic Biol Med 29:1222–1233 [DOI] [PubMed]
- 151.Shen SC, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC (2002) In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production. Eur J Pharmacol 446:187–194 [DOI] [PubMed]
- 152.Shirai M, Moon JH, Tsushida T, Terao J (2001) Inhibitory effect of a quercetin metabolite, quercetin 3-O-beta-d-glucuronide, on lipid peroxidation in liposomal membranes. J Agric Food Chem 49:5602–5608 [DOI] [PubMed]
- 153.Simpson L, Parsons R (2001) PTEN: life as a tumor suppressor. Exp Cell Res 264:29–41 [DOI] [PubMed]
- 154.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK (1996) Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer 26:167–181 [DOI] [PubMed]
- 155.Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ (2000) Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol 58:535–541 [DOI] [PubMed]
- 156.Spencer JPE, Abd El Mohsen MM, Rice-Evans C (2004) Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys 423: 148–161 [DOI] [PubMed]
- 157.Spencer JPE, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C (1999) The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett 458:224–230 [DOI] [PubMed]
- 158.Spencer JPE, Kuhnle GG, Williams RJ, Rice-Evans C (2003) Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem J 372:173–181 [DOI] [PMC free article] [PubMed]
- 159.Spencer JPE, Rice-Evans C, Williams RJ (2003) Modulation of pro-survival Akt/PKB and ERK1/2 signalling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 278:34783–34793 [DOI] [PubMed]
- 160.Spencer JPE, Schroeter H, Crossthwaithe AJ, Kuhnle G, Williams RJ, Rice-Evans C (2001a) Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radic Biol Med 31:1139–1146 [DOI] [PubMed]
- 161.Spencer JPE, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, Rice-Evans C (2001b) Epicatechin and its in vivo metabolite, 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem J 354:493–500 [DOI] [PMC free article] [PubMed]
- 162.Spencer JPE, Schroeter H, Rechner AR, Rice-Evans C (2001c) Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal 3:1023–1039 [DOI] [PubMed]
- 163.Spencer JPE, Srai SK, Rice-Evans C (2003d) Metabolism in the small intestine and gastrointestinal tract. In: Rice-Evans C, Packer L (eds). Flavonoids in health and disease. Marcel Dekker, New York, pp 363–390
- 164.Sperker B, Backman JT, Kroemer HK (1997) The role of beta-glucuronidase in drug disposition and drug targeting in humans. Clin Pharmacokinet 33:18–31 [DOI] [PubMed]
- 165.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112–116 [DOI] [PubMed]
- 166.Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H (1998) Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19:1771–1776 [DOI] [PubMed]
- 167.Sweatt JD (2001) Memory mechanisms: the yin and yang of protein phosphorylation. Curr Biol 11:R391–R394 [DOI] [PubMed]
- 168.Talavera S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Remesy C (2005) Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem 53:3902–3908 [DOI] [PubMed]
- 169.Tatton WG, Olanow CW (1999) Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta 1410:195–213 [DOI] [PubMed]
- 170.Terao J, Yamaguchi S, Shirai M, Miyoshi M, Moon JH, Oshima S, Inakuma T, Tsushida T, Kato Y (2001) Protection by quercetin and quercetin 3-O-beta-d-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radic Res 35:925–931 [DOI] [PubMed]
- 171.Tobiume K, Saitoh M, Ichijo H (2002) Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol 191:95–104 [DOI] [PubMed]
- 172.Torres M, Forman HJ (2003) Redox signaling and the MAP kinase pathways. Biofactors 17:287–296 [DOI] [PubMed]
- 173.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T (1999) Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 274:2234–2242 [DOI] [PubMed]
- 174.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J (2002) Redox control of cell death. Antioxid Redox Signal 4:405–414 [DOI] [PubMed]
- 175.Unno K, Takabayashi F, Kishido T, Oku N (2004) Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp Gerontol 39:1027–1034 [DOI] [PubMed]
- 176.Ursini F, Maiorino M, Morazzoni P, Roveri A, Pifferi G (1994) A novel antioxidant flavonoid (IdB 1031) affecting molecular mechanisms of cellular activation. Free Radic Biol Med 16:547–553 [DOI] [PubMed]
- 177.Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S (2001) The role of glial cells in Parkinson’s disease. Curr Opin Neurol 14:483–489 [DOI] [PubMed]
- 178.Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248 [PubMed]
- 179.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6:909–919 [DOI] [PubMed]
- 180.Walle T, Walgren RA, Walle UK, Galijatovic A, Vaidyanathan JB (2003) Understanding the bioavailability of flavanoids through studies in Caco-2 cells. In: Rice-Evans C, Packer L (eds) Flavonoids in health and disease. Marcel Dekker, New York, pp 349–362
- 181.Wang L, Matsushita K, Araki I, Takeda M (2002) Inhibition of c-Jun N-terminal kinase ameliorates apoptosis induced by hydrogen peroxide in the kidney tubule epithelial cells (NRK-52E). Nephron 91:142–147 [DOI] [PubMed]
- 182.Wang XS, Diener K, Jannuzzi D, Trollinger D, Tan TH, Lichenstein H, Zukowski M, Yao Z (1996) Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem 271:31607–31611 [DOI] [PubMed]
- 183.Wang Y, Wang L, Wu J, Cai J (2006) The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol 148:147–153 [DOI] [PMC free article] [PubMed]
- 184.Weston CR, Lambright DG, Davis RJ (2002) Signal transduction. MAP kinase signaling specificity. Science 296:2345–2347 [DOI] [PubMed]
- 185.Williams RJ, Spencer JPE, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36:838–849 [DOI] [PubMed]
- 186.Woo KJ, Lim JH, Suh SI, Kwon YK, Shin SW, Kim SC, Choi YH, Park JW, Kwon TK (2006) Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology 211:359–368 [DOI] [PubMed]
- 187.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL (1998) The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 95:15587–15591 [DOI] [PMC free article] [PubMed]
- 188.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331 [DOI] [PubMed]
- 189.Yamamoto N, Moon JH, Tsushida T, Nagao A, Terao J (1999) Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch Biochem Biophys 372:347–354 [DOI] [PubMed]
- 190.Yamauchi T (2005) Neuronal Ca2+/calmodulin-dependent protein kinase II-discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull 28:1342–1354 [DOI] [PubMed]
- 191.Yang SH, Galanis A, Sharrocks AD (1999) Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol 19:4028–4038 [DOI] [PMC free article] [PubMed]
- 192.Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z (2000) Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res 60:4053–4057 [PubMed]
- 193.Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem 85:180–192 [DOI] [PubMed]
- 194.Youdim KA, Joseph JA (2001) A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med 30:583–594 [DOI] [PubMed]
- 195.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ (2004a) Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic Biol Med 36:592–604 [DOI] [PubMed]
- 196.Youdim KA, Shukitt-Hale B, Joseph JA (2004b) Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med 37:1683–1693 [DOI] [PubMed]
- 197.Youdim KA, Spencer JP, Schroeter H, Rice-Evans C (2002) Dietary flavonoids as potential neuroprotectants. Biol Chem 383:503–519 [DOI] [PubMed]
- 198.Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809 [DOI] [PubMed]
- 199.Zervos AS, Faccio L, Gatto JP, Kyriakis JM, Brent R (1995) Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates Max protein. Proc Natl Acad Sci USA 92:10531–10534 [DOI] [PMC free article] [PubMed]
- 200.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ (1996) Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628 [DOI] [PubMed]
- 201.Zhang L, Chen J, Fu H (1999) Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci USA 96:8511–8515 [DOI] [PMC free article] [PubMed]
- 202.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di PF, Olson EN, Ulevitch RJ, Han J (1999) Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol 19:21–30 [DOI] [PMC free article] [PubMed]
- 203.Zimmermann M, Colciaghi F, Cattabeni F, Di Luca M (2002) Ginkgo biloba extract: from molecular mechanisms to the treatment of Alzhelmer’s disease. Cell Mol Biol 48:613–623 [PubMed]