Abstract
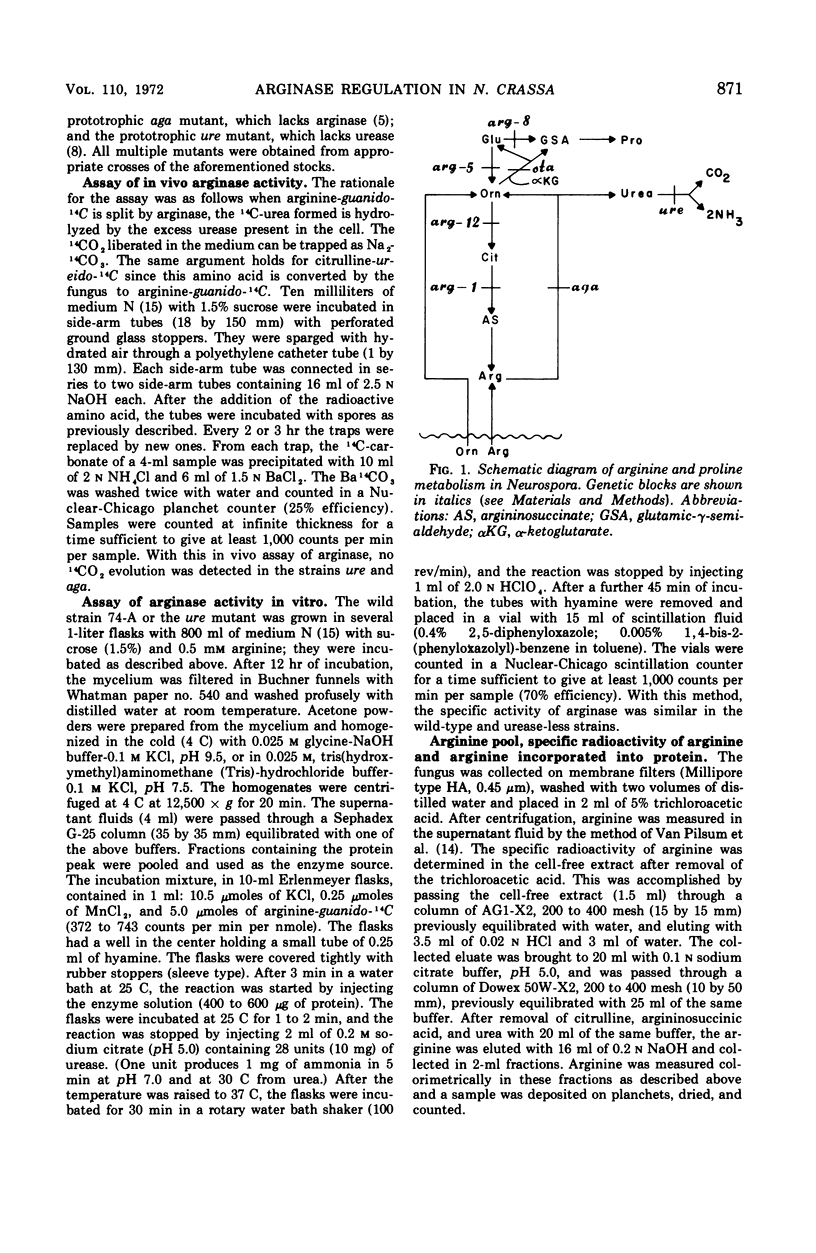
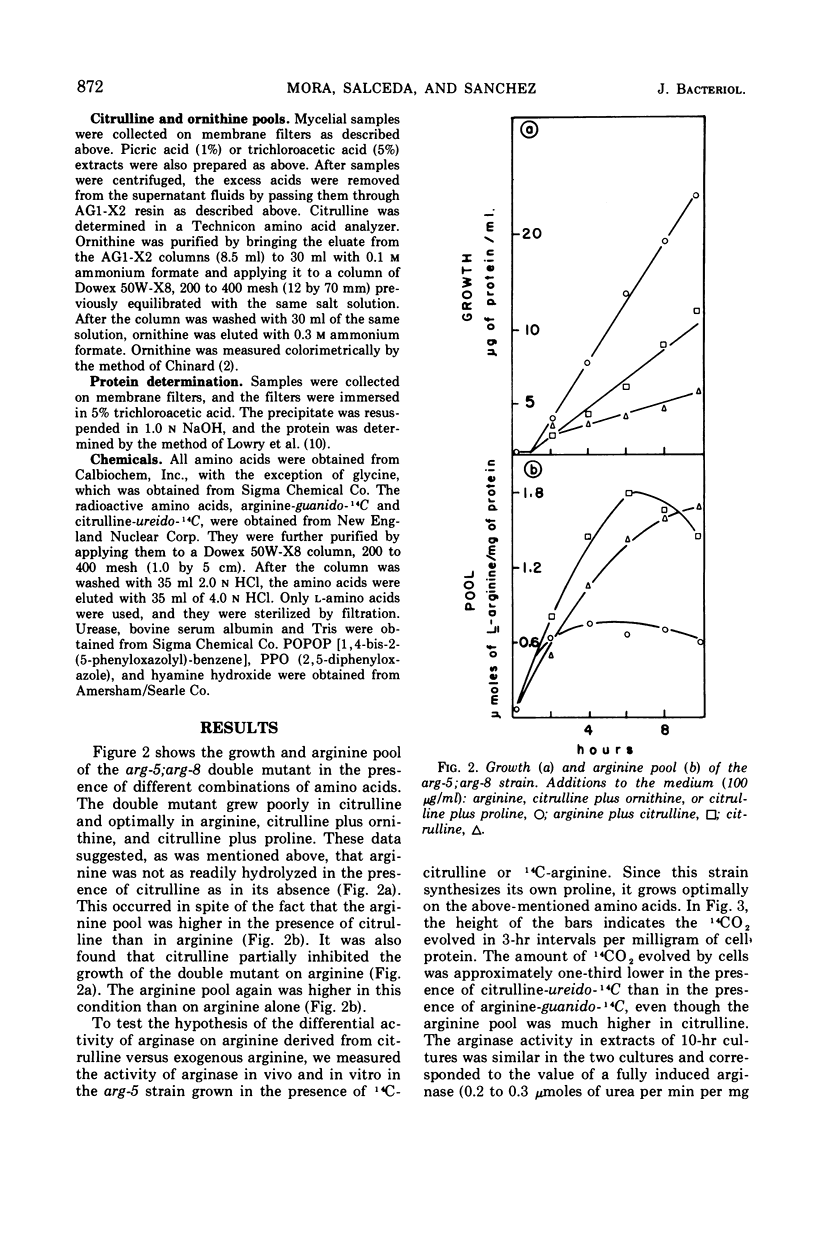
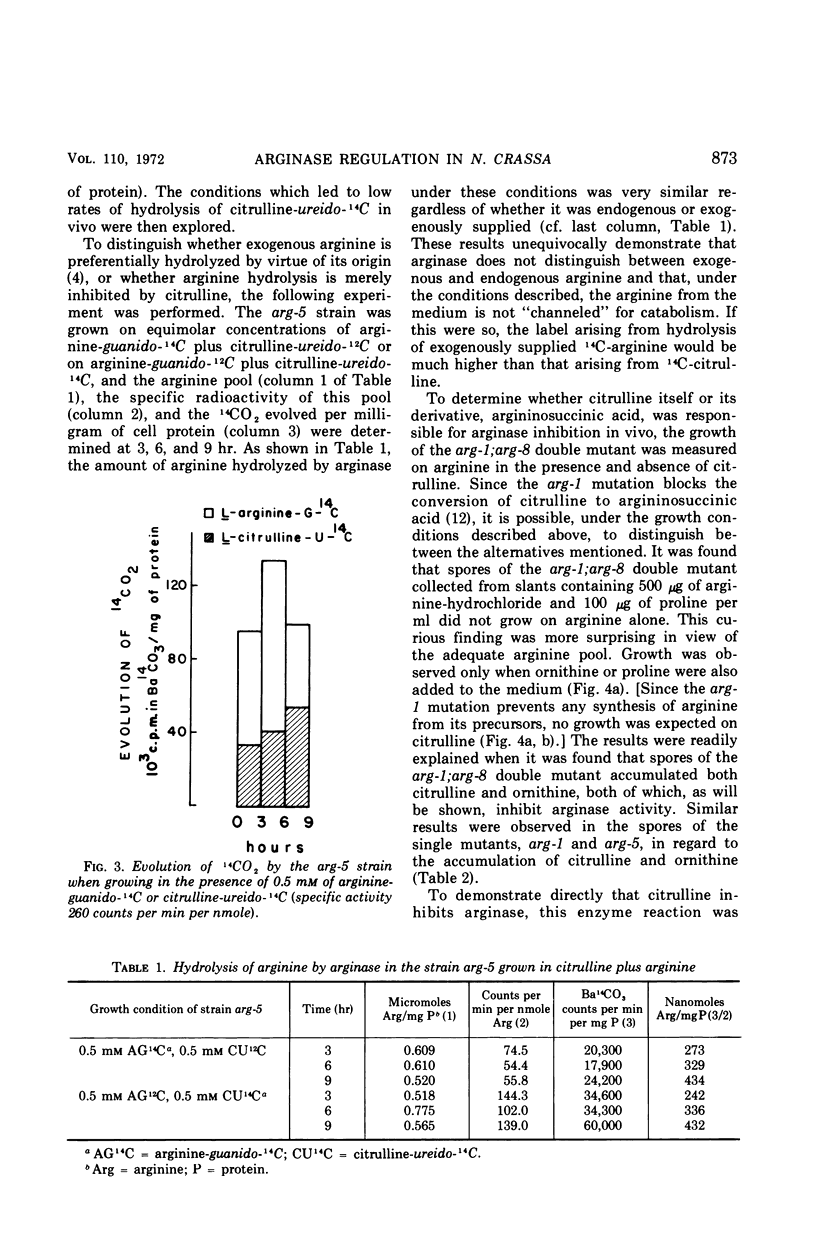
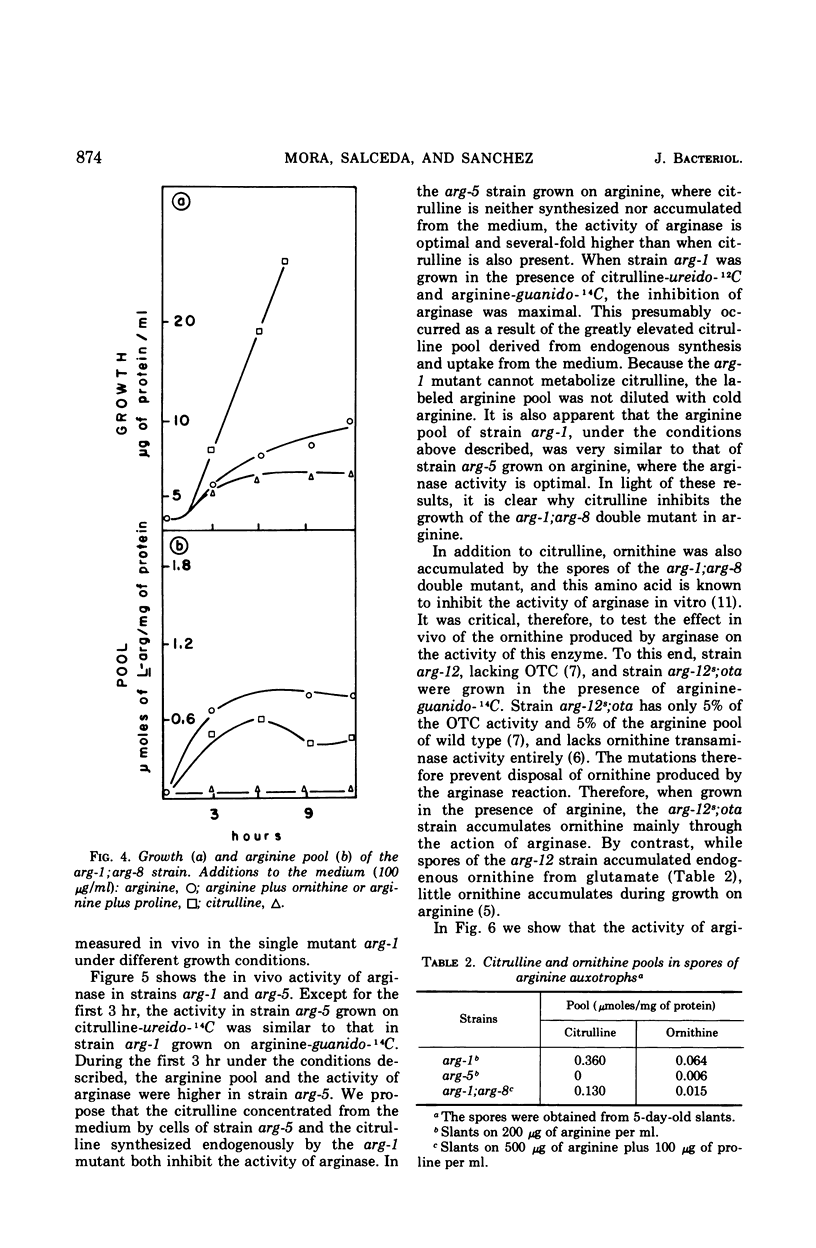
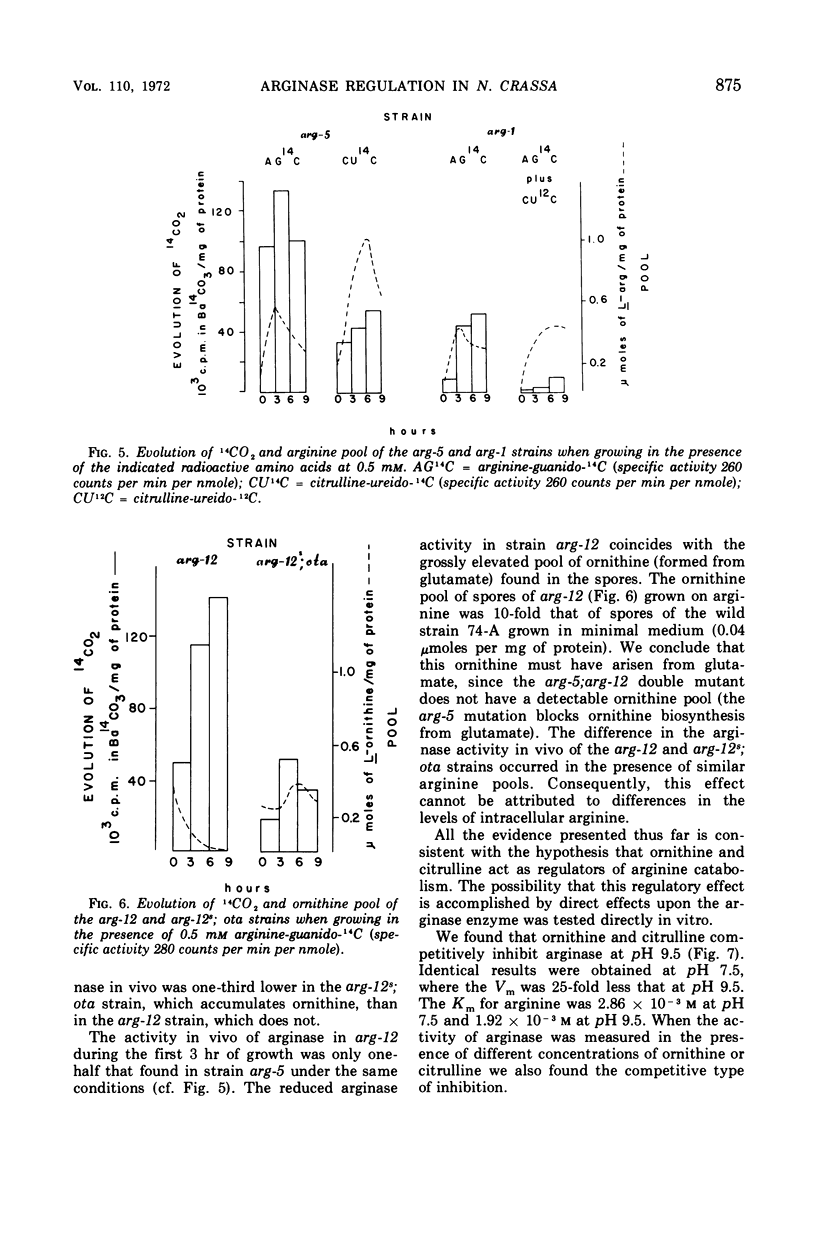
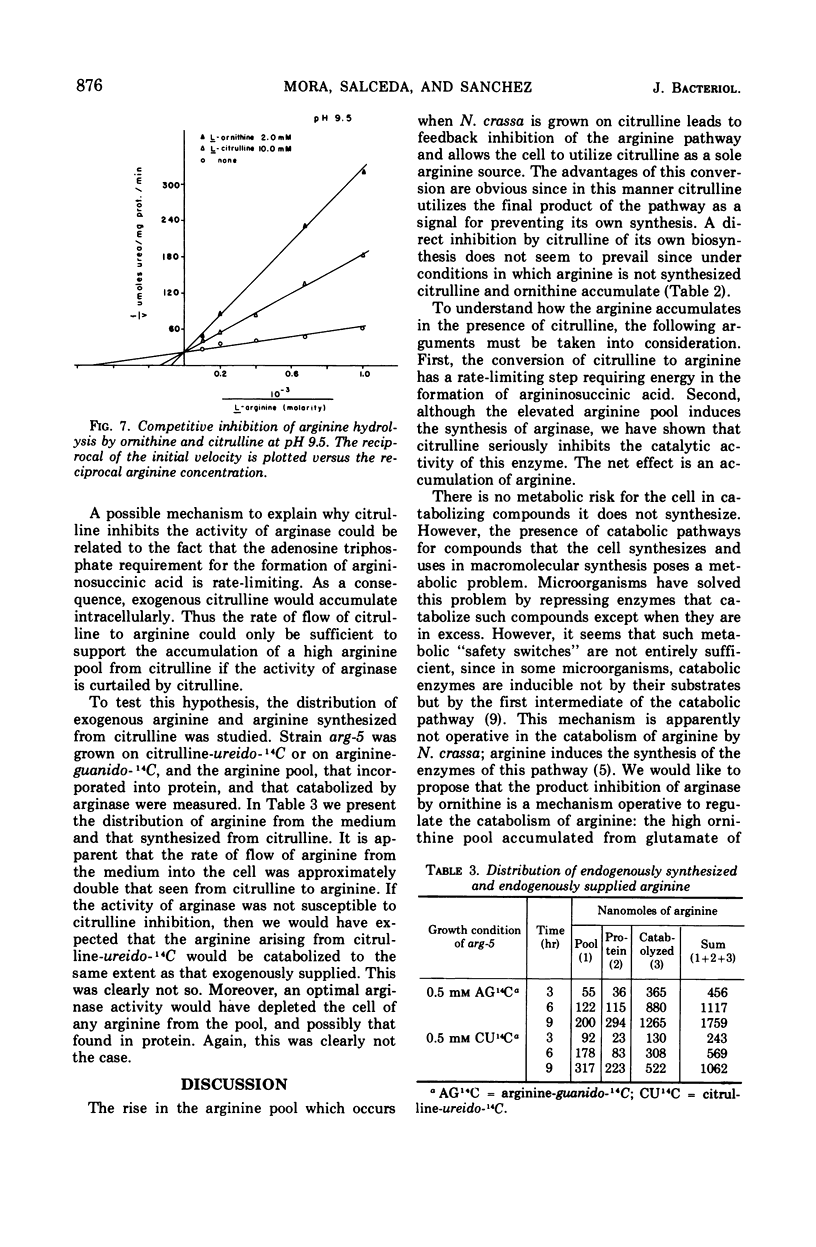
It has been found that, in Neurospora crassa, arginine synthesized from exogenous citrulline was not as effectively hydrolyzed as exogenous arginine. This was explained by the observed inhibition of arginase in vitro and in vivo by citrulline. The high arginine pool formed from exogenous citrulline feedback inhibits the arginine pathway. These two factors allow exogenous citrulline to be used adventitiously and efficiently as an arginine source. Finally, it was found that ornithine was a strong inhibitor of arginase. This suggests that the characteristically high ornithine pool of minimal cultures of Neurospora may act to control a potentially wasteful catabolism of endogenous arginine by arginase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- Castañeda M., Martuscelli J., Mora J. The catabolism of L-arginine by Neurospora crassa. Biochim Biophys Acta. 1967 Jul 25;141(2):276–286. doi: 10.1016/0304-4165(67)90102-x. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H., THWAITES W. M. STRUCTURAL GENE FOR ORNITHINE TRANSCARBAMYLASE IN NEUROSPORA. Genetics. 1963 Nov;48:1551–1558. doi: 10.1093/genetics/48.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mora J., Tarrab R., Bojalil L. F. On the structure and function of different arginases. Biochim Biophys Acta. 1966 Apr 12;118(1):206–209. doi: 10.1016/s0926-6593(66)80161-3. [DOI] [PubMed] [Google Scholar]
- NEWMEYER D. Arginine synthesis in Neurospora crassa; Genetic studies. J Gen Microbiol. 1957 Apr;16(2):449–462. doi: 10.1099/00221287-16-2-449. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. II. Properties of a basic amino acid transport system. Biochim Biophys Acta. 1970 Mar 17;203(1):139–149. doi: 10.1016/0005-2736(70)90044-1. [DOI] [PubMed] [Google Scholar]
- Vogel H. J., Bonner D. M. ON THE GLUTAMATE-PROLINE-ORNITHINE INTERRELATION IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1954 Aug;40(8):688–694. doi: 10.1073/pnas.40.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]


