Abstract
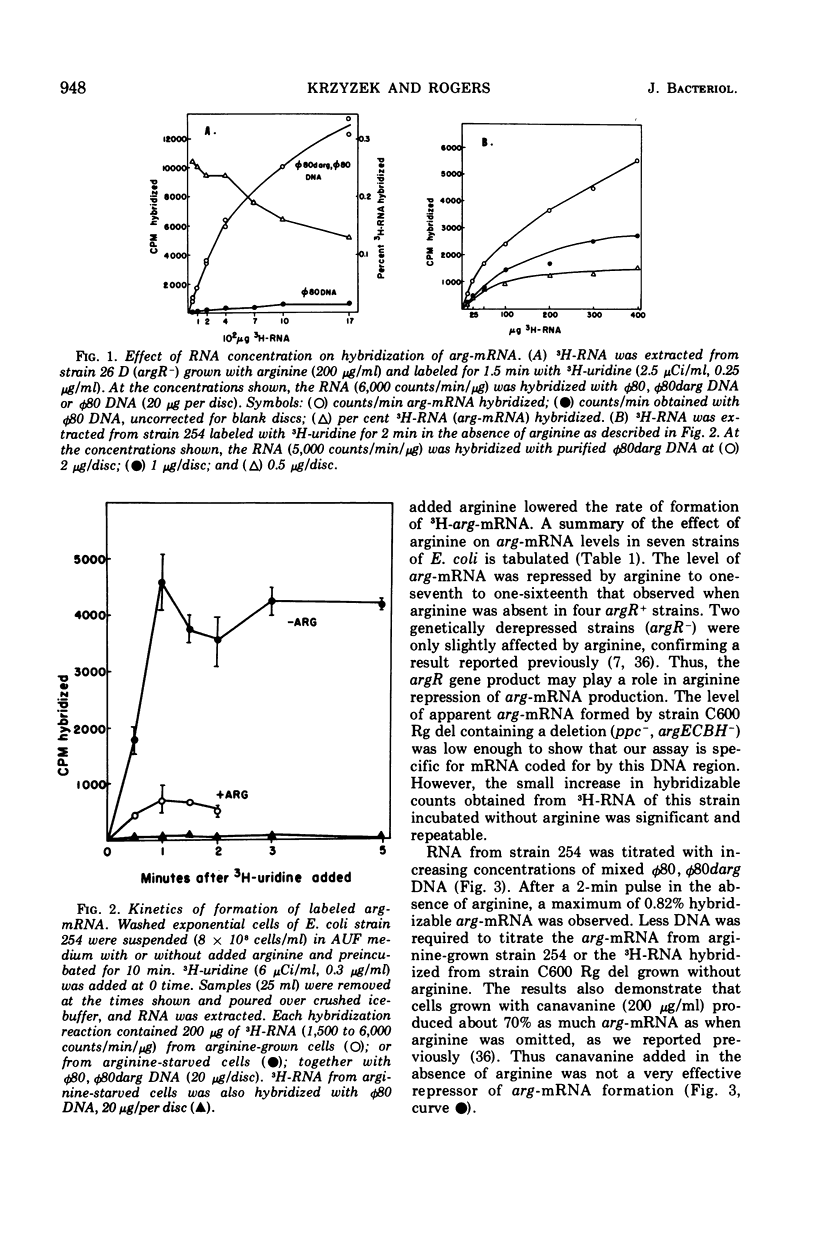
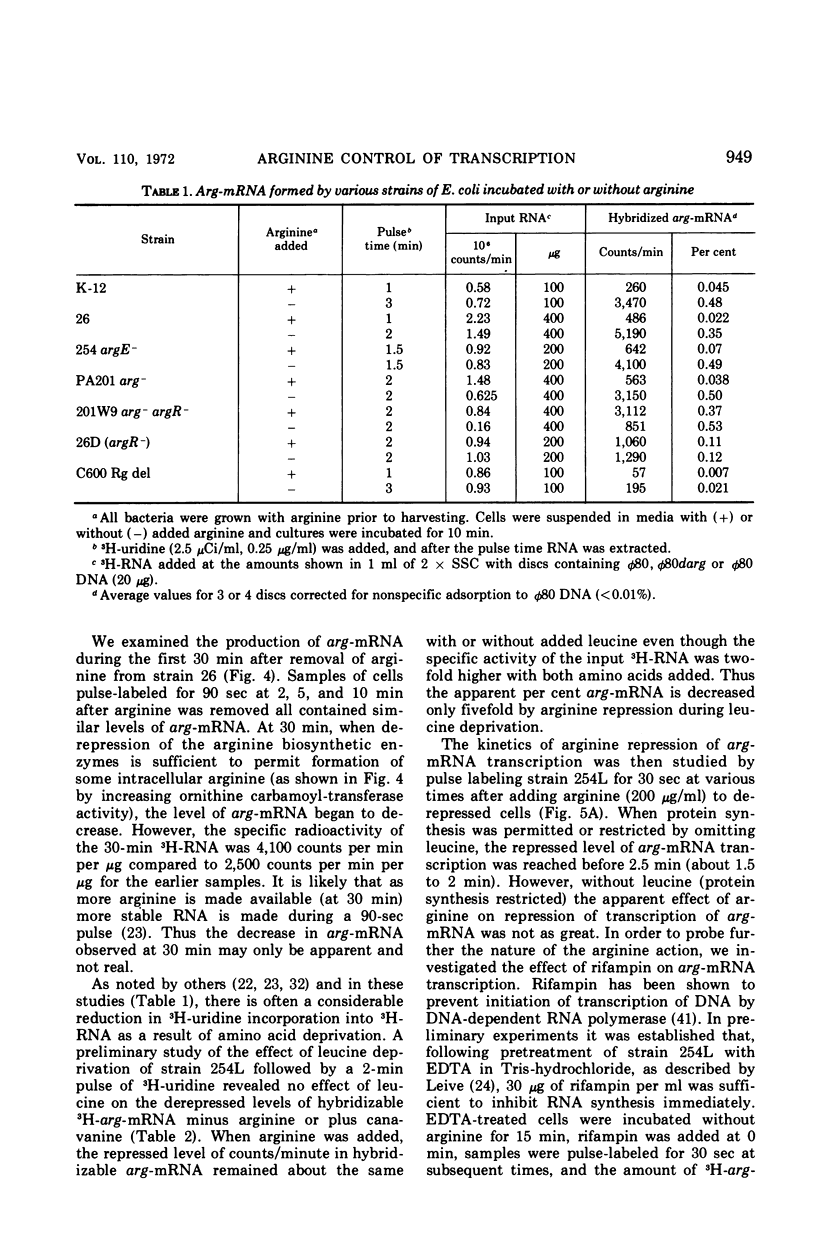
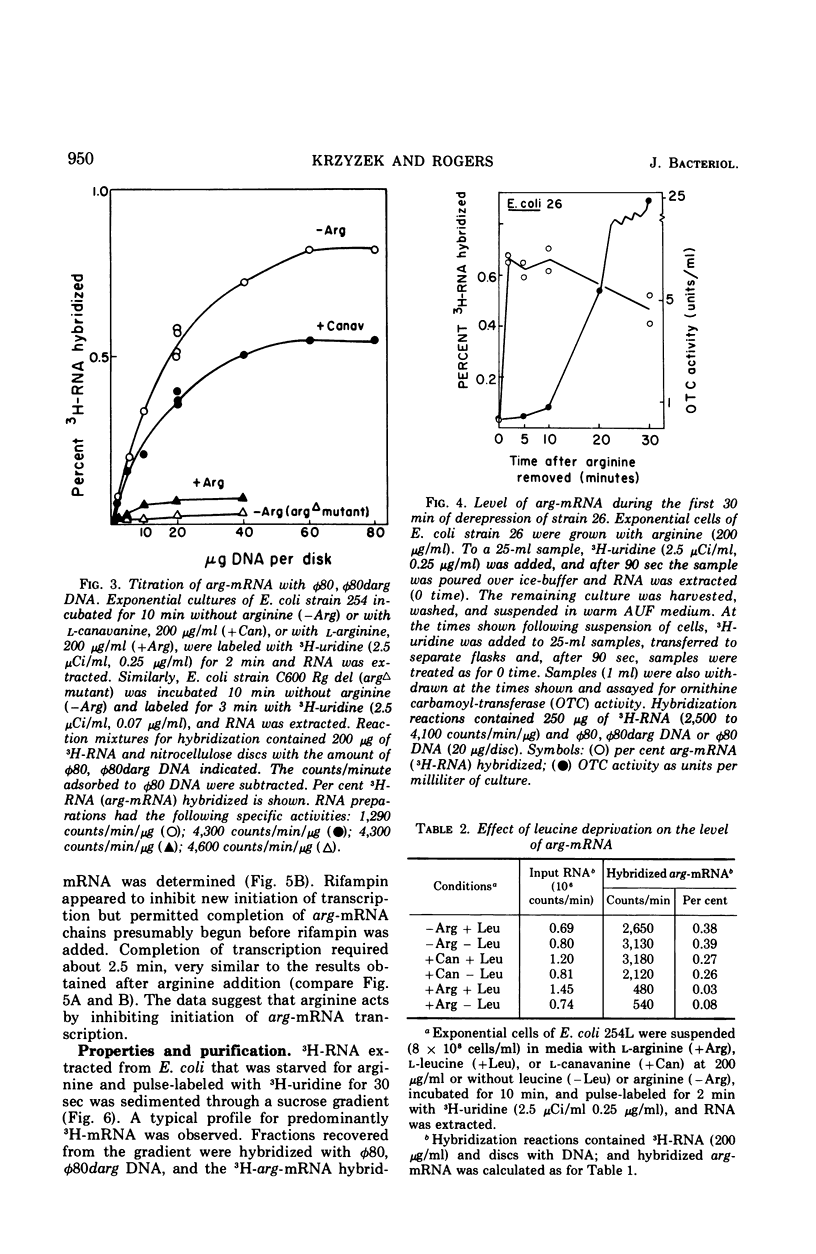
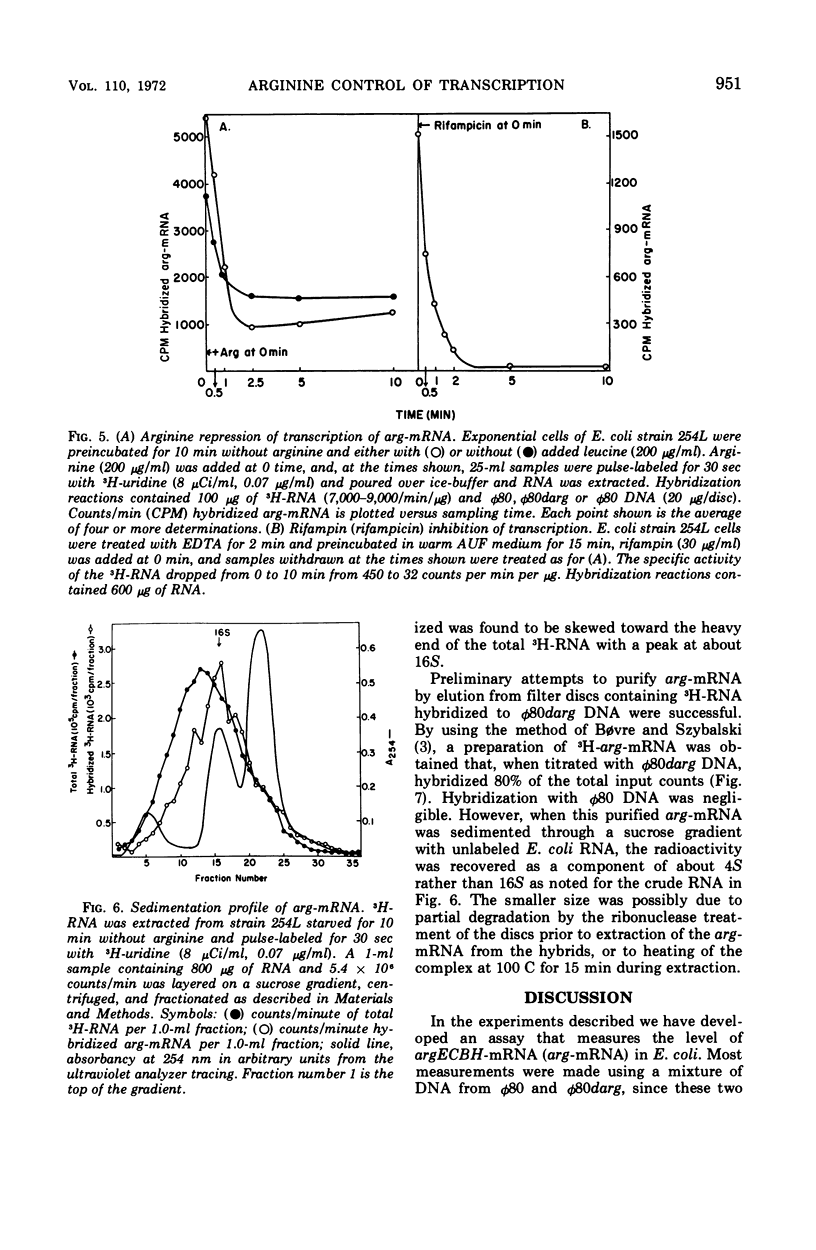
The level of messenger ribonucleic acid specific for the argECBH gene cluster (arg-mRNA) of Escherichia coli was measured by deoxyribonucleic acid-ribonucleic acid hybridization in a number of strains. During the first 10 min after removal of arginine (derepression), the rate of arg-mRNA accumulation was six to ten times greater than that found in arginine-repressed argR+ cells. In the absence of arginine, l-canavanine (200 μg/ml) repressed arg-mRNA synthesis to a level only 20 to 30% lower than that found after arginine deprivation. High levels of arg-mRNA were produced by argR− strains with or without added arginine. Within about 2 min after arginine addition to argR+ cells, the rate of synthesis of arg-mRNA reached the repressed level. Likewise, 2.5 min after rifampin addition, all transcription of arg-mRNA was completed. These data are consistent with the view that arginine signals repression by inhibiting the initiation of transcription of arg-mRNA mediated in some way by the argR gene. The kinetics of arg-mRNA accumulation and the kinetics of completion of transcription together with the profile of hybridizable arg-mRNA in sucrose density gradients (major component 16S) suggest that the argECBH gene cluster is transcribed in short pieces rather than as a single unit.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coyne B. J. Depression kinetics of ornithine transcarbamylase in Escherichia coli. Biophys J. 1970 Oct;10(10):911–936. doi: 10.1016/s0006-3495(70)86343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Elseviers D., Sand G., Freundlich G., Glandsdorff N. On the functional organization of the arg ECBH cluster of genes in Escherichia coli K-12. Mol Gen Genet. 1969;106(1):32–47. doi: 10.1007/BF00332819. [DOI] [PubMed] [Google Scholar]
- Cunin Raymond, Glansdorff Nicolas. Messenger RNA from arginine and phosphoenolpyruvate carboxylase genes in arg R+ and arg R(-) strains of E. coli K-12. FEBS Lett. 1971 Oct 15;18(1):135–137. doi: 10.1016/0014-5793(71)80428-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hirvonen A. P., Vogel H. J. Response of argR- spheroplasts of Escherichia coli to extracted arginine repressor. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1611–1616. doi: 10.1016/0006-291x(70)90573-5. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Morikawa N., Sato K., Mishima S., Nishimura T. On the transcription of the tryptophan operon in Escherichia coli. II. Production of the specific messenger RNA. J Mol Biol. 1965 Aug;13(1):157–168. doi: 10.1016/s0022-2836(65)80086-9. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Morikawa N., Sato K. On the transcription of the tryptophan operon in Escherichia coli. 3. Multicistronic messenger RNA and polarity for transcription. J Mol Biol. 1965 Aug;13(1):169–182. doi: 10.1016/s0022-2836(65)80087-0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Yanofsky C. Transcription of the tryptophan operon in polarity mutants of Escherichia coli. II. Evidence for normal production of tryp-mRNA molecules and for premature termination of transcription. J Mol Biol. 1967 Aug 28;28(1):25–35. doi: 10.1016/s0022-2836(67)80074-3. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- Karlström O., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. II. Application to the physiological evidence. J Mol Biol. 1969 Jan 14;39(1):89–94. doi: 10.1016/0022-2836(69)90335-0. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Lavallé R. Regulation at the level of translation in the arginine pathway of Escherichia coli K12. J Mol Biol. 1970 Jul 28;51(2):449–451. doi: 10.1016/0022-2836(70)90154-3. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- MAAS W. K., MAAS R., WIAME J. M., GLANSDORFF N. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. I. DOMINANCE OF REPRESSIBILITY IN ZYGOTES. J Mol Biol. 1964 Mar;8:359–364. doi: 10.1016/s0022-2836(64)80199-6. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Transcription of the tryptophan operon in Escherichia coli: rifampicin as an inhibitor of initiation. J Mol Biol. 1970 Mar;48(3):525–531. doi: 10.1016/0022-2836(70)90064-1. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO K., SUGINO Y., NOMURA M. Synthesis and turnover of phage messenger RNA in E. coli infected with bacteriophage T4 in the presence of chloromycetin. J Mol Biol. 1962 Nov;5:527–534. doi: 10.1016/s0022-2836(62)80126-0. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Formation of ornithine transcarbamylase in cells and protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Jun;33(2):423–436. doi: 10.1016/0006-3002(59)90132-5. [DOI] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- Udaka S. Isolation of the arginine repressor in Escherichia coli. Nature. 1970 Oct 24;228(5269):336–338. doi: 10.1038/228336a0. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Singer J., Heffernan L. Deoxyribonucleic acid-ribonucleic acid hybridization studies on the L-Arabinose operon of Escherichia coli B-r. J Bacteriol. 1971 Oct;108(1):1–4. doi: 10.1128/jb.108.1.1-4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


