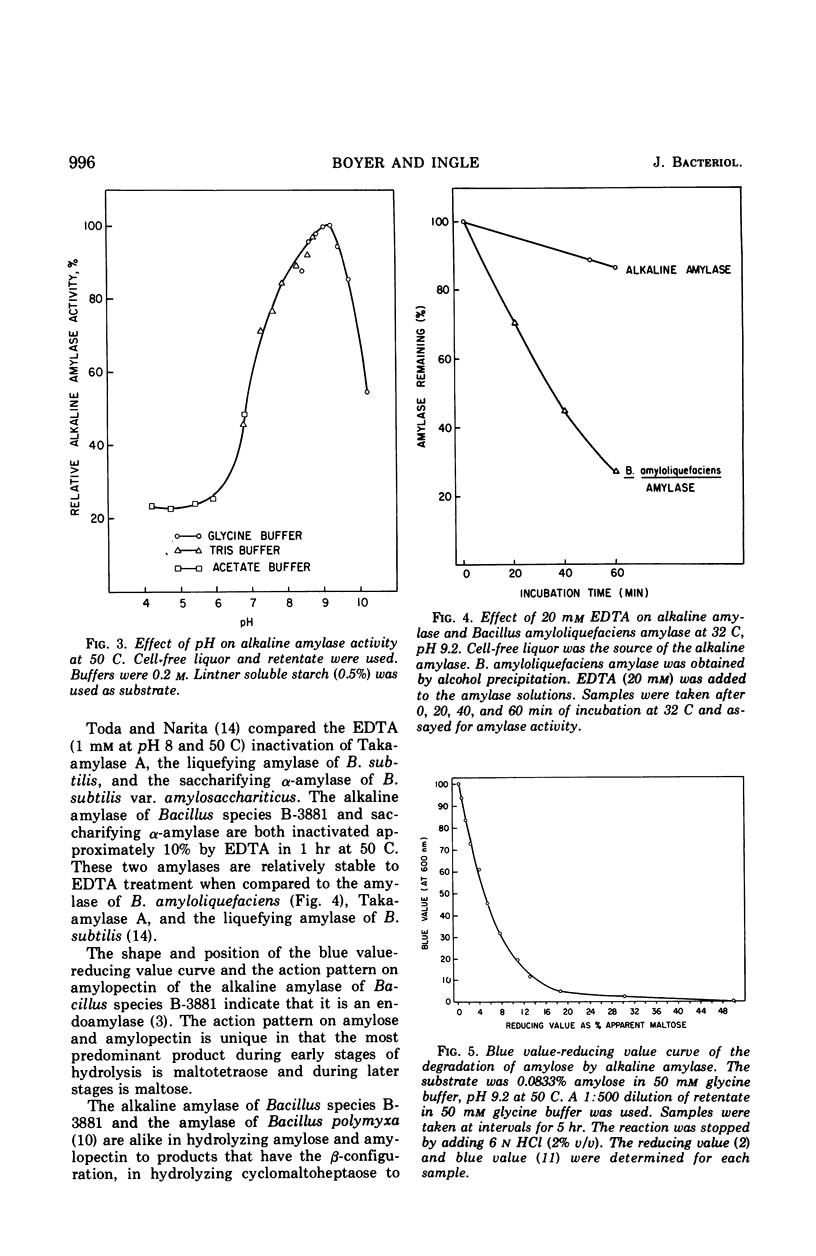
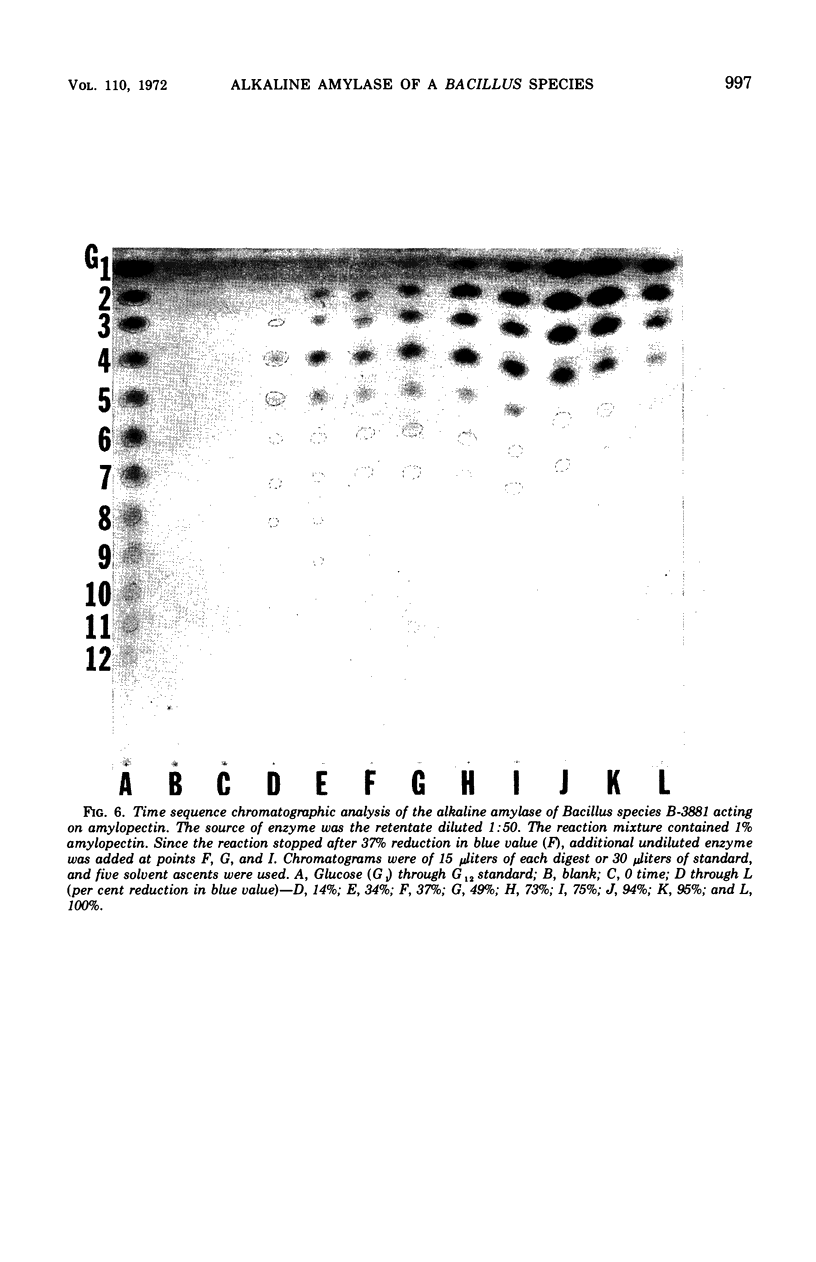
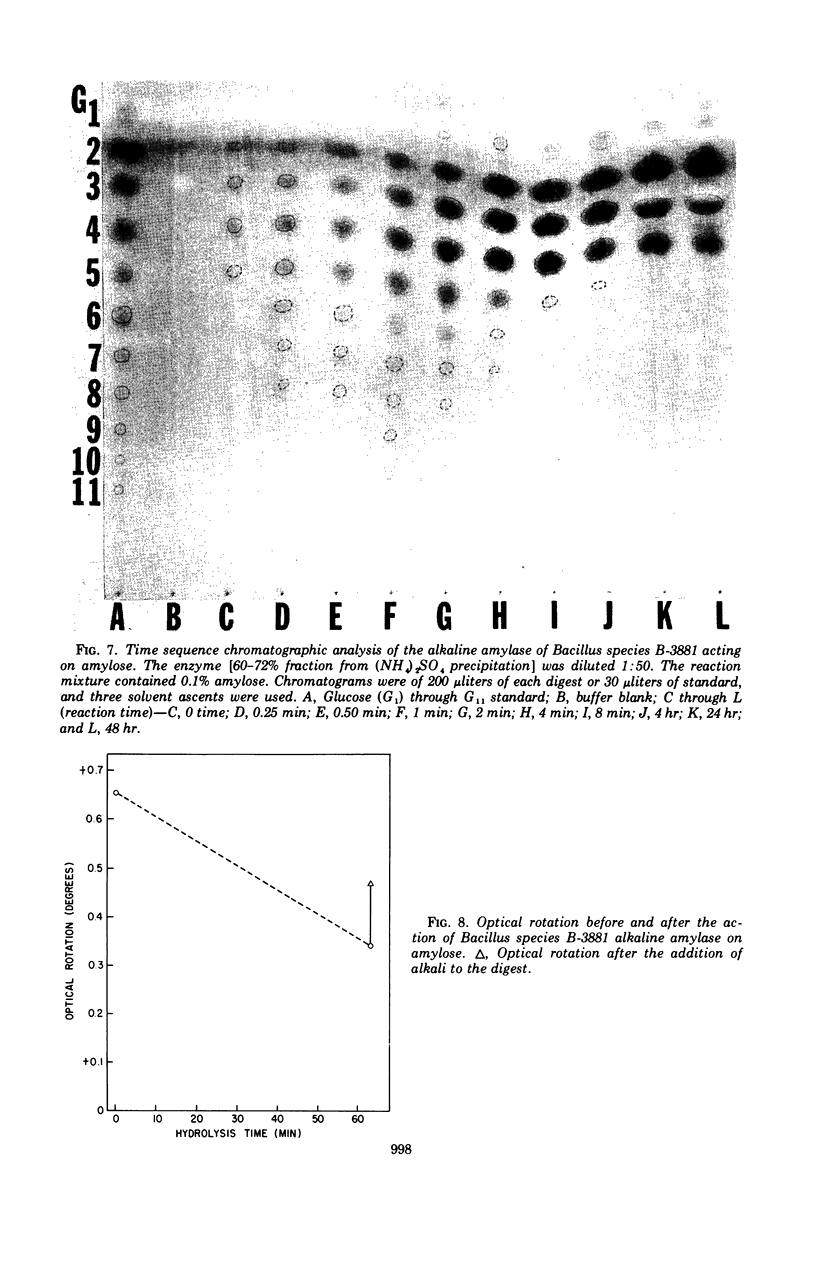
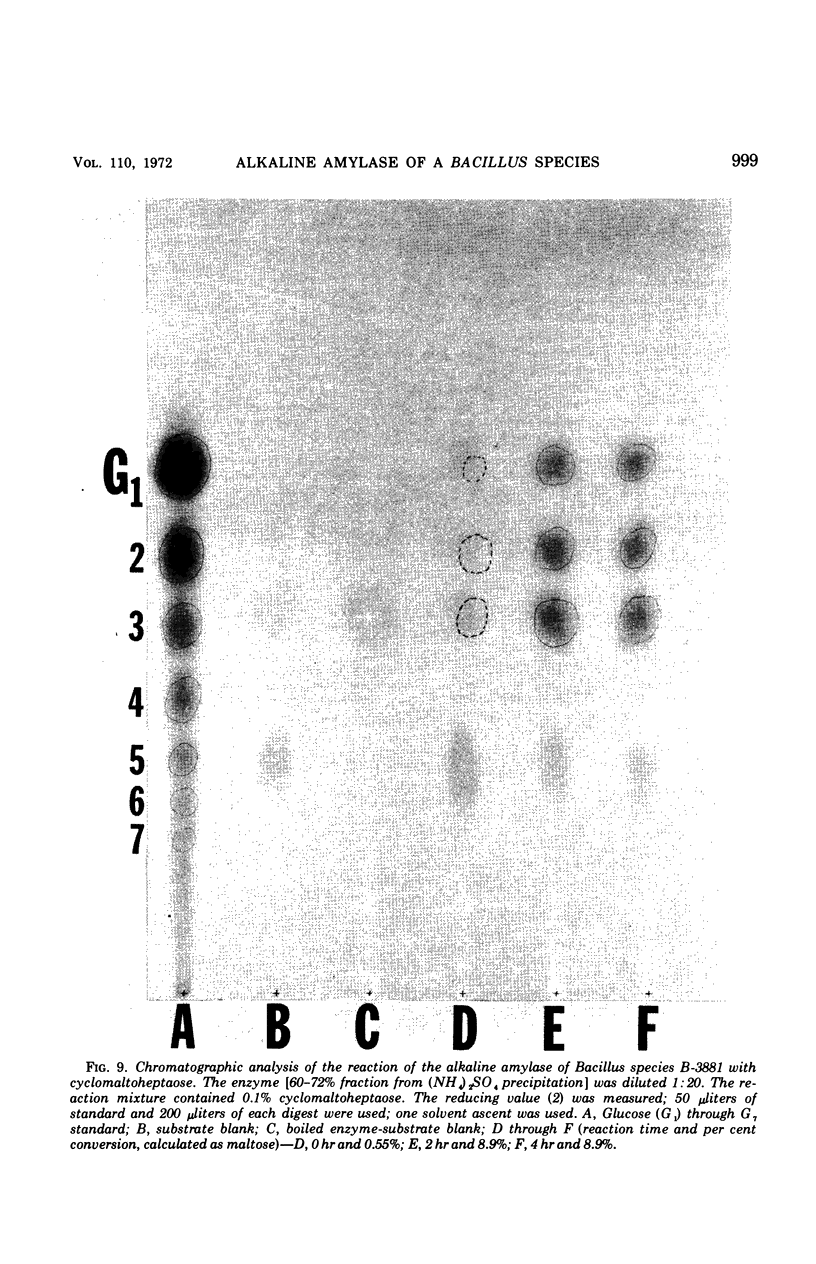
Abstract
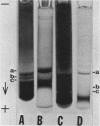
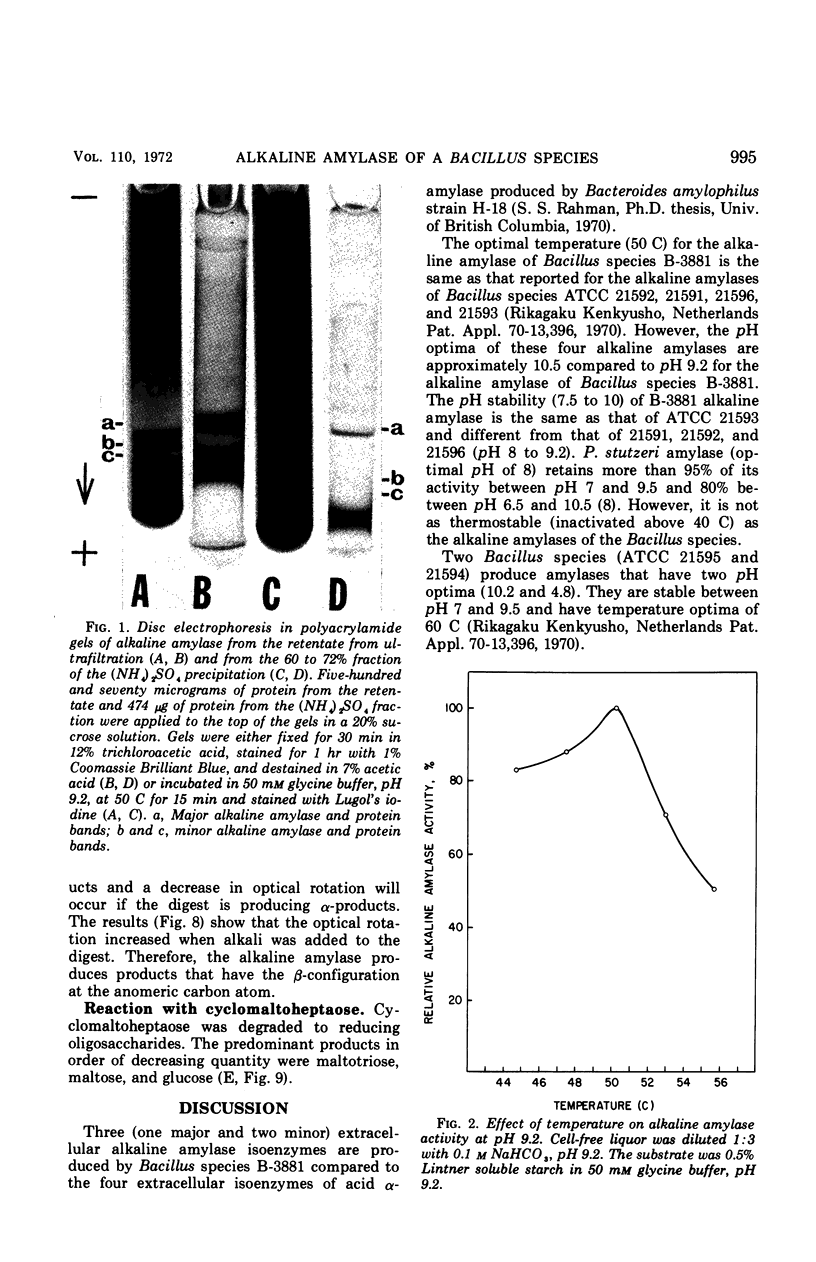
A selective medium was used to isolate a bacterium (Bacillus species NRRL B-3881) that produced extracellular alkaline amylase in an alkaline medium (pH 9.5). Maximal enzyme yield was obtained in an aerated medium after 21 hr at 36 C. The enzyme was purified 18-fold by ultrafiltration and ammonium sulfate precipitation. Three active isoenzymes (one major and two minor) of alkaline amylase were detected by disc electrophoresis in polyacrylamide gel. The enzyme was only 12% inactivated by 20 mm ethylenediaminetetraacetic acid after 1 hr at pH 9.2 and 32 C. The optimal temperature was 50 C at pH 9.2, and the optimal pH was 9.2 at 50 C. The enzyme was stable between pH 7.5 and 10. It had an endomechanism of substrate encounter. The products produced from amylose and amylopectin had the β-configuration. Cyclomaltoheptaose was hydrolyzed to maltotriose, maltose, and glucose. The main final product produced from amylose and amylopectin was β-maltose; the other final products were maltotriose and small quantities of glucose and maltotetraose. The predominant product at early stages of hydrolysis was maltotetraose; other products were maltose through maltonanaose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. PURIFICATION AND ACTION PATTERN OF AN AMYLASE FROM BACILLUS POLYMYXA. Arch Biochem Biophys. 1964 Feb;104:338–345. doi: 10.1016/s0003-9861(64)80024-2. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J. Isolation, purification, and characterization of a maltotetraose-producing amylase from Pseudomonas stutzeri. Arch Biochem Biophys. 1971 Jul;145(1):105–114. doi: 10.1016/0003-9861(71)90015-4. [DOI] [PubMed] [Google Scholar]
- Sather B. T. A comparative study of amylases and proteinases in some decapod crustacea. Comp Biochem Physiol. 1969 Jan;28(1):371–379. doi: 10.1016/0010-406x(69)91350-4. [DOI] [PubMed] [Google Scholar]
- Toda H., Narita K. Correlation of the sulfhydryl group with the essential calcium in Bacillus subtilis saccharifying alpha-amylase. J Biochem. 1968 Mar;63(3):302–307. [PubMed] [Google Scholar]