Abstract
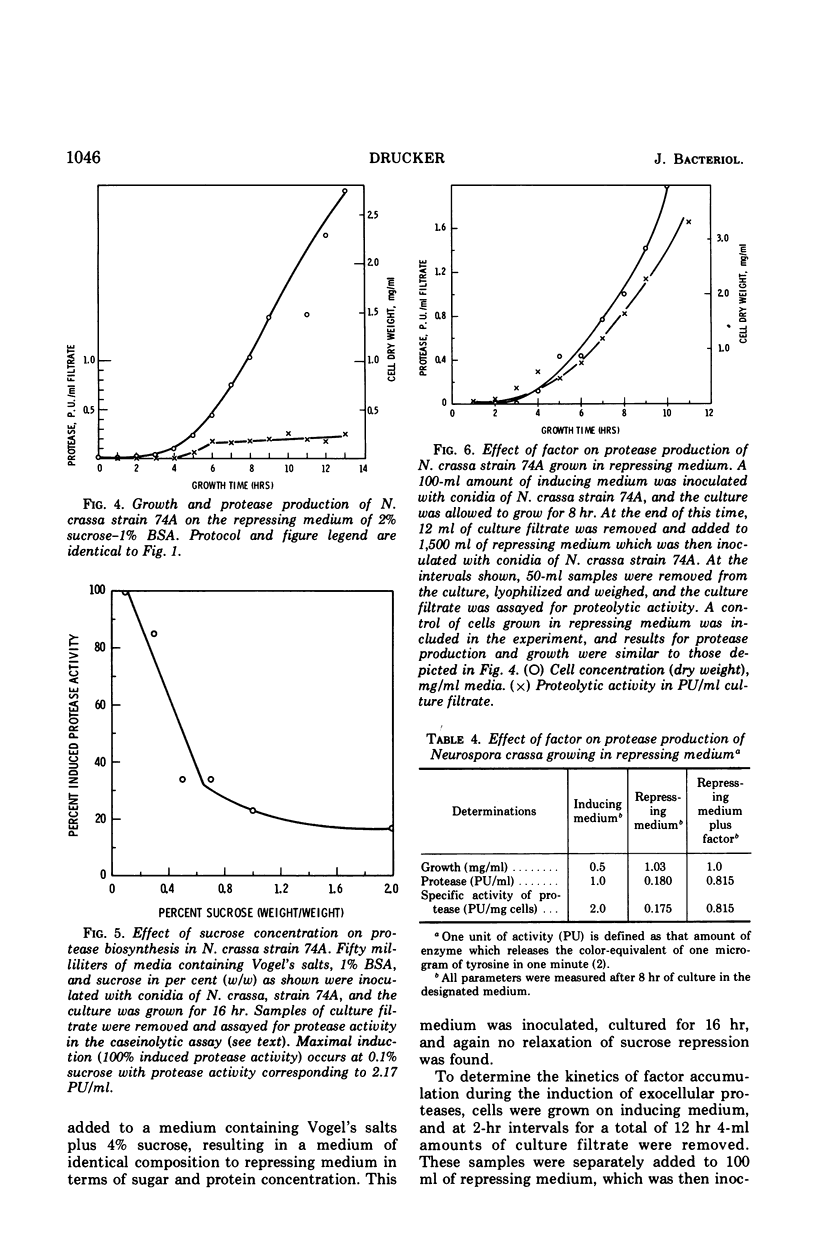
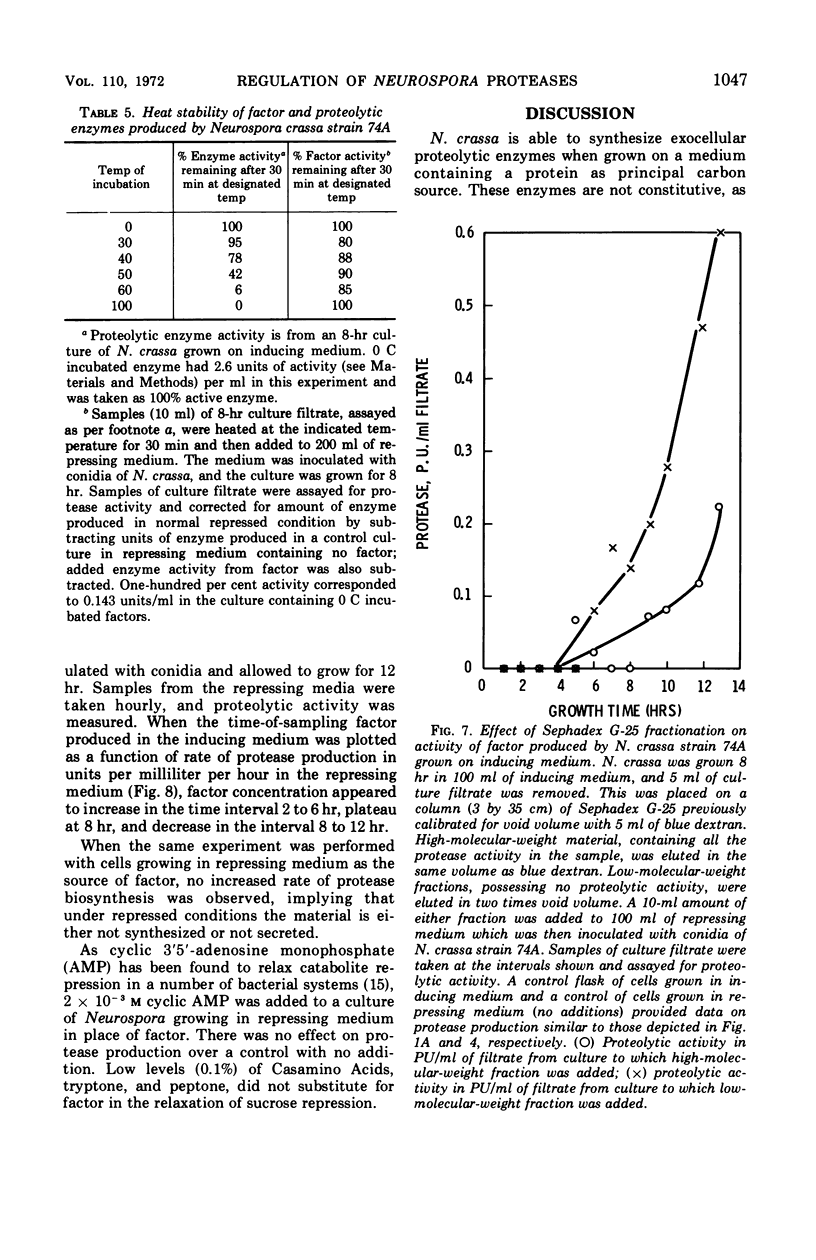
Neurospora crassa strain 74A grown on Vogel's medium containing bovine serum albumin (BSA) as principal carbon source secretes proteolytic enzymes which appear in the culture filtrate. Low concentrations of sucrose (0.1%) are necessary for growth from conidia, as conidia will not germinate on BSA alone. Once growth is initiated, however, protease production begins and at 5 to 6 hr growth and enzyme production are parallel. Higher concentrations of sucrose (0.5-2%) repress protease synthesis. Other metabolizable materials (sugars, amino acids, peptide mixtures) also repress protease synthesis. Some sugars will not sustain growth but allow germination and full induction of protease in the presence of protein. A material found in culture fluids of cells during induction of protease synthesis when added to repressed cultures causes a five-fold increase in the amount of protease production, although this is still approximately half that of normally induced cells. This material appears to be produced by induced cells in as little as 2 hr of culture, which is before detectable levels of protease can be found. It is heat-stable, of low molecular weight, and is not a simple product of protein digestion by the N. crassa proteases.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ito M., Sugiura M. [Studies on Aspergillus proteinase. Pueification, crystallization and some properties of alkaline proteinase from Aspergillus melleus (studies on enzymes. XXXVII)]. Yakugaku Zasshi. 1968 Dec;88(12):1576–1582. doi: 10.1248/yakushi1947.88.12_1576. [DOI] [PubMed] [Google Scholar]
- Levisohn S., Aronson A. I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967 Mar;93(3):1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Multiple proteolytic enzymes of Streptomyces fradiae. Production, isolation, and preliminary characterization. Biochim Biophys Acta. 1967 Jul 11;139(2):382–397. doi: 10.1016/0005-2744(67)90042-3. [DOI] [PubMed] [Google Scholar]
- Morihara K. Production of proteinase on noncarbohydrate carbon sources by Pseudomonas aeruginosa. Appl Microbiol. 1965 Sep;13(5):793–797. doi: 10.1128/am.13.5.793-797.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVAL J. J., NICKERSON W. J. Decomposition of native keratin by Streptomyces fradiae. J Bacteriol. 1959 Mar;77(3):251–263. doi: 10.1128/jb.77.3.251-263.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi Y., Shibuya K., Yanagita M. Studies on proteolytic enzymes (pronase) of Streptomyces griseus K-1. II. Separation of exo- and endopeptidases of pronase. J Biochem. 1968 Oct;64(4):427–437. doi: 10.1093/oxfordjournals.jbchem.a128914. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J., Mathison G. E. The localization and secretion of a propteolytic enzyme complex by the dermatophytic fungus Microsporum canis. J Gen Microbiol. 1971 Nov;68(3):319–326. doi: 10.1099/00221287-68-3-319. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Transcription and degradation of messenger ribonucleic acid for a glucose transport system in Neurospora. J Biol Chem. 1971 Aug 10;246(15):4784–4789. [PubMed] [Google Scholar]
- Sierra G. Germination of bacterial endospores with subtilopeptidases. Can J Microbiol. 1967 May;13(5):489–501. doi: 10.1139/m67-064. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Sorsoli W. A., Matchett W. H. Induction of kynureninase in Neurospora. J Bacteriol. 1970 Aug;103(2):364–369. doi: 10.1128/jb.103.2.364-369.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


