Abstract
Small GTPases of the Ras-like (Ral) family are crucial for signalling functions in both normal and cancer cells; however, their role in a developing organism is poorly understood. Here, we identify the Drosophila Ral homologue RalA as a new key regulator of polar-cell differentiation during oogenesis. Polar cells have a crucial role in patterning the egg chamber and in recruiting border cells, which undergo collective and guided migration. We show that RalA function is essential for the maintenance of anterior and posterior polar-cell fate and survival. RalA is required cell autonomously to control the expression of polar-cell-specific markers, including the Jak/Stat ligand Unpaired. The loss of RalA also causes a cell non-autonomous phenotype owing to reduced Jak/Stat signalling in neighbouring follicle cells. As a result, border-cell assembly and migration as well as the polarization of the oocyte are defective. Thus, RalA is required in organizing centres to control proper patterning and migration in vivo.
Keywords: RalA, polar cell, border cell, Jak/Stat
Introduction
The migration of border cells (BCs) is a powerful model system to study collective migration, which occurs during normal development and also in some epithelial cancers. BCs migrate as a cohort of cells with mixed epithelial and mesenchymal attributes. Each cluster is made up of two cell types organized radially with two central polar cells (PCs) encircled by 6–8 outer border cells (oBCs; Fig 1A,B; Rorth, 2002; Montell, 2003). PCs have been shown to be crucial for organizing function in egg chambers (Grammont & Irvine, 2002; Xi et al, 2003), whereas oBCs are involved in the migratory activity of BCs. Because the organization of BC clusters is functionally important and dynamic (Bianco et al, 2007; Prasad & Montell, 2007), it is crucial to identify the genes that control PC and oBC differentiation and to understand how these groups of cells are assembled during development, as well as to explain how the coordination of assembly and migration is carried out.
Figure 1.
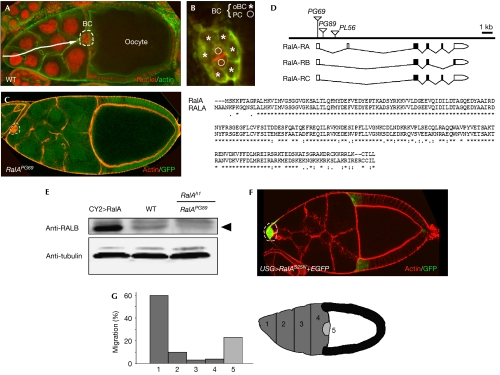
RalA controls border-cell migration in egg chambers. (A) Wild-type (WT) stage-10 egg chamber stained with phalloidin (green) and propidium iodide (red). Border cells (BCs; enclosed by dotted lines) have reached the anterior region of the oocyte, after delaminating from the anterior epithelium and migrating through the nurse-cell compartment (arrow). (B) BC cluster organization showing two centrally located polar cells (PCs; circles) surrounded by six outer BCs (oBCs; asterisks). (C) Mosaic stage-10 egg chamber containing homozygous RalAPG69 cells (GFP–negative). Note the absence of migration of BCs (enclosed by dotted lines). Actin is shown in red. (D) Schematic view of the genomic organization of the RalA locus and ClustalW alignment of RalA and human RALA (accession no. EAL23994) protein sequences. (E) Western blot of ovary extracts. RalA (black arrowhead) is detected using a polyclonal antibody directed against the human RALB protein. Tubulin antibodies were used to assess protein levels in each lane. (F) Expression of UAS–RalAS25N in BCs (enclosed by dotted lines) blocks their migration. (G) Stage-10 migration defects following expression of RalAS25N in BCs. The nurse-cell compartment (dark grey) has been divided into five regions (1–5) to determine the extent of migration. GFP, green fluorescent protein.
Results And Discussion
To isolate new genes involved in BC organization and migration, we conducted a genetic mosaic screen in Drosophila egg chambers using a set of 311 independent P-element-mediated lethal mutations, targeting individual genes on the underscreened X chromosome (Bourbon et al, 2002; Ghiglione et al, 2002; see Methods). One mutation, PG69, induced a complete lack of BC migration (Fig 1C). The determination of PG69 chromosomal position showed that it is inserted in the 5′ untranslated region of RalA/CG2849, the unique Drosophila homologue of human RAL genes (Fig 1D). Another insertion, PG89, in the first intron of RalA (Fig 1D) was analysed and showed phenotypes similar to those of PG69 (data not shown). As PG69 and PG89 are Gal4-expressing enhancer-trap lines (Bourbon et al, 2002), they were used to drive expression of diverse UAS lines in a RalA mutant background. Expression of UAS–RalA (a wild-type form of RalA) or UAS–RalAG20V (a constitutively activated form; Sawamoto et al, 1999) rescued lethality and migration (Fig 2E; 29 out of 30 rescued egg chambers showed normal migration). However, expression of UAS–RalAS25N (a dominant-negative form; Sawamoto et al, 1999) did not, indicating that PG69 and PG89 are mutations targeting the RalA gene. Furthermore, a crossreacting polyclonal antibody directed against the human RALB protein allowed detection of RalA in wild type, which was strongly reduced in mutant ovaries taken from a viable RalAh1/RalAPG89 combination (Fig 1E).
Figure 2.
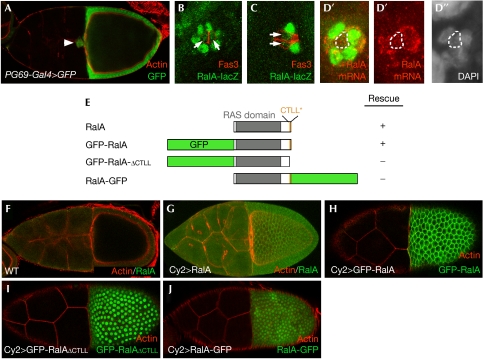
Expression of RalA and sequence determinants for RalA subcellular localization. (A) Expression of RalA in border cells (BCs) and follicle cells was detected using a RalA–Gal4 enhancer trap (RalAPG69) crossed to UAS–GFP. Expression of RalA–LacZ in BCs (B) during or (C) when migration has completed using the RalAPL56 enhancer-trap line. Expression of β-galactosidase is detected in outer BCs (oBCs) as well as in polar cells (PCs). Fas3 staining is used to mark PCs (red). (D–D″) Fluorescent in situ hybridization of a stage-9 egg chamber using an antisense RalA probe, showing expression in PCs and oBCs. Red indicates RalA mRNA and green EYA (marks oBCs but not PCs, enclosed by dotted lines). (E) Schematic representation of RalA wild-type and GFP fusion proteins, showing the RAS domain and the CTLL motif at the C terminus. Each RalA construct was used in rescue experiments to assess their activity (see text for details). (F) The human RALB antibody (Fig 1E) does not detect endogenous levels of RalA expression but recognizes overexpressed wild-type RalA (using CY2–Gal4), which localizes to the plasma membrane (G). GFP–RalA expressed using CY2–Gal4 also localizes to the plasma membrane (H). (I) GFP–RalAΔCTLL, in which the last four C-terminal amino acids are deleted, is mislocalized and accumulated predominantly in the nucleus. (J) RalA–GFP fusion protein is also mislocalized in the nucleus. GFP, green fluorescent protein.
Overexpression of UAS–RalAS25N, but not UAS–RalA, in BCs induced by using USG–Gal4 (a combination of two Gal4 lines, Upd–Gal4 and slbo–Gal4) led to a high proportion of BC clusters that did not migrate at all or migrated only slightly (>70%, n=298; Fig 1F,G), thereby confirming our genetic clonal analysis. Consistently, RalA is expressed in follicle and border cells, as shown by in situ hybridization and specific enhancer-trap lines (Fig 2A–D″). The RalA protein is localized to the plasma membrane in ovaries and we showed that a carboxy-terminal sequence containing a putative CAAX box (198-CTLL-STOP), important for prenylation of H-Ras and other small GTPases, is essential for RalA subcellular localization and function. Indeed, the deletion or hiding of this motif was sufficient to abolish both the rescue activity and plasma membrane localization of RalA (Fig 2E,H–J).
RalA has been shown to be downstream from Drosophila Rap1 for bristle development (Mirey et al, 2003). We thus tested whether Rap1 might also be crucial in egg chambers. Interestingly, expression of a dominant-negative form of Rap1 (Rap1N17) blocked migration of BCs, similar to RalAS25N (compare Fig 1 and supplementary Fig 2 online), suggesting that Rap1 and RalA are part of a common pathway for BC migration.
To determine which cells among the BC cluster might require RalA function, we analysed mosaic clusters containing zero, one or two mutant PCs with wild-type or mutant oBCs. We found that the BC cluster formed and migrated normally when all oBCs were mutant for RalA (Fig 3A–B′), indicating that RalA function is not required in oBCs. Consistently, mosaic analysis showed an essential function of RalA in PCs, with a varied phenotype depending on the number of RalA mutant PCs present in the BC cluster. When only one of the two PCs was mutant, the BC formed but did not migrate (Figs 1C, 3C–D′). In this case, the number of oBCs was reduced with an average of 3.5±0.7 oBCs (n=26) recruited instead of 6.1±0.9 in wild type (n=24; see the table in Fig 3). These data indicate that one PC cannot compensate for the loss of the other to recruit a full set of oBCs. As one PC has been shown to be sufficient for BC migration, these results indicate that the presence of a RalA mutant PC inhibits migration. In egg chambers in which both PCs were mutant for RalA, almost no oBCs were recruited, with an average of 0.6±0.6 oBC per cluster (n=18; Fig 3E–F′). This result was confirmed using targeted expression of RalA RNA-mediated interference specifically in PCs using the Upd–Gal4 driver (data not shown). The lack or reduction of oBCs was confirmed using terminal follicle cell markers, including slbo–lacZ and E(Spl)–lacZ enhancer-trap expression (Fig 4G–H′; supplementary Fig 1 online). Note that the expression of these oBC markers remained unaffected when only oBCs were mutant for RalA (data not shown), indicating a cell non-autonomous function of RalA in PCs to control recruitment of oBCs and migration of the cluster.
Figure 3.
Drosophila RalA is essential in polar cells for border-cell recruitment and migration. (A) Mosaic border-cell (BC) cluster in which the two polar cells (PCs; arrows in B,B′) are wild type, whereas outer BCs (oBCs; enclosed by dotted lines) are mutant for RalAPG69. Mutant cells are negative for GFP (green). PCs are marked by Fas3 (red). Stage-10 egg chamber is shown. (B,B′) Enlargement of the boxed region shown in (A). (C) BC cluster in which only one PC is wild type (arrow; GFP and Fas3 positive) does not migrate. Early stage-10 egg chamber is shown. (D,D′) Enlargement of the boxed region shown in (C). (E) The two PCs are mutant in this egg chamber and BCs did not form. (F,F′) Enlargement of the boxed region shown in (E). Stage-10A egg chambers are shown in (A,C) and a stage-10B egg chamber is shown in (E). Green indicates GFP clonal marker and grey 4′,6-diamidino-2-phenylindole (DAPI) staining. The dotted lines indicate clonal limits. The table shows a summary of RalA-induced BC phenotypes shown in (A–F′). Left column, number of wild-type PCs; middle column, number of recruited oBCs; right column, migration phenotype (+, wild type; −, no migration). GFP, green fluorescent protein.
Figure 4.
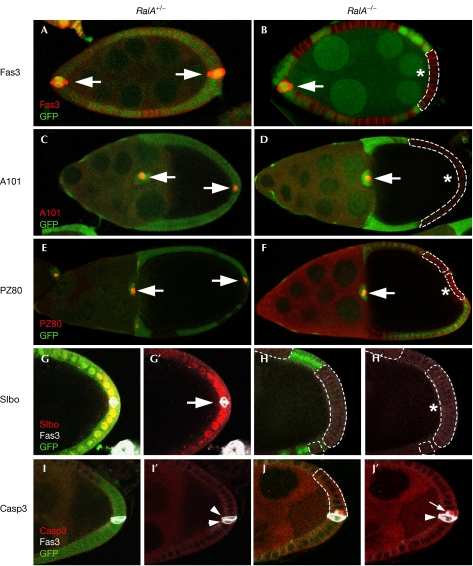
Drosophila RalA controls polar-cell and terminal follicle cell differentiation. Specific markers for polar cells (PCs; Fas3, A101–lacZ, PZ80–lacZ) and terminal follicle cells (Slbo–lacZ) were used to analyse cell fate in RalA homozygous follicle cells (B,D,F,H–H′,J–J′), as compared with heterozygous cells (A,C,E,G–G′,I–I′). The expression of Fas3 (A,B), A101–lacZ (C,D) and PZ80–lacZ (E,F) is completely lost when PCs are mutant for RalA. The RalA mutations also lead to a complete loss of expression of the terminal follicle cell marker Slbo–lacZ (G–G′,H–H′). PCs that are mutant for RalA show a specific activation of Casp3 (J–J′; in red; white arrow) and this is not observed in wild-type PCs at this late stage (I–I′; white arrowheads). Green indicates GFP clonal marker and grey Fas3 staining (G–J′). The dotted lines indicate clonal limits and the asterisks mutant PCs. GFP, green fluorescent protein.
To analyse further the role of RalA in PC function, we stained egg chambers using a set of PC-specific markers. These markers are expressed both at the anterior and posterior poles, so clones made at either side of the egg chamber have been analysed indifferently.
When anterior or posterior PCs were mutant for RalA, the expression of Fas3, PZ80 and A101 were abolished, indicating that RalA is essential for proper PC differentiation (Fig 4A–F). The effect of RalA mutations on Fas3 protein was stage specific: before stage 6–7, Fas3 staining was normal, whereas later on, staining was strongly reduced or absent, suggesting that RalA controls the maintenance of PC differentiation.
It has been shown that PCs originate from a larger group of pre-PCs in which cell number is reduced from 3–5 to 2 through apoptosis at early stages (Besse & Pret, 2003). To assess whether de-differentiated PCs could initiate apoptosis, we stained egg chambers with antibodies raised against activated caspase 3 (Casp3). Interestingly, some of the RalA mutant PCs (20%, n=30), but not other follicle cells, induced strong activated Casp3 expression (Fig 4I–J′), which is indicative of cells undergoing cell death. As not all the mutant PCs showed activated Casp3 expression, this suggests that either this expression is transient or mutant PCs might show distinct behaviours. It is interesting to note that RalA was recently shown to protect cells from apoptosis in the sensory lineage (Balakireva et al, 2006), suggesting that this gene might have an anti-apoptotic role in differentiated cells during Drosophila development. Altogether, our results identify RalA as a new regulator to maintain PC fate and survival.
RalA shows a cell non-autonomous phenotype originating from the PC. PCs are essential anteriorly for recruiting a ring of around six oBCs that make a mature BC cluster, which depends on the secretion of the Unpaired (Upd) ligand from the PC and subsequent Jak/Stat activation in the oBC (Silver & Montell, 2001; Beccari et al, 2002; Ghiglione et al, 2002; McGregor et al, 2002). Secretion of Upd and binding to Domeless (Dome), the Drosophila Jak/Stat receptor, induces ligand-dependent internalization of Dome in cells surrounding PCs, both in BCs and posterior follicle cells (Fig 5A,A′; Ghiglione et al, 2002; Devergne et al, 2007). When PCs were mutant for RalA, Dome-containing endocytic vesicles were no longer observed, both anteriorly and posteriorly (Fig 5B,B′), suggesting that Jak/Stat signalling was not activated. In wild-type egg chambers, Stat is localized in the nucleus as a gradient, with higher levels of nuclear Stat close to the PC (Fig 5C,C′), thus reflecting Jak/Stat pathway activation. The nuclear localization of Stat was normal when PCs were wild type with adjacent follicle cells mutant for RalA (Fig 5D,D′), indicating that RalA does not have a role in the function of oBCs and posterior follicle cells in controlling Jak/Stat signalling. By contrast, in egg chambers with mutant PCs, Stat nuclear localization was completely abolished (Fig 5E). To discriminate between a role of RalA in Upd expression or activity, we stained egg chambers using a Upd antibody, which shows a gradient of this ligand in egg chambers (Fig 5F). Using this assay, we showed that RalA mutations in PCs strongly affect the expression of the Upd protein (Fig 5G).
Figure 5.
RalA mutant polar cells block Jak/Stat signalling and oocyte anterior–posterior polarization. (A,A′) Mosaic egg chamber in which the two anterior polar cells (PCs) are wild type (enclosed by dotted lines). Domeless (Dome; in red) is found in intracellular vesicles (arrows). (B,B′) Mosaic egg chamber in which the two anterior PCs are mutant for RalA (asterisk). Note the absence of Dome endocytic vesicles. Mutant cells do not express the clonal marker GFP (green). PCs are marked with Fas3 (green). (C,C′) Graded nuclear expression of Stat in a heterozygous RalA+/− egg chamber. (D,D′) Expression of Stat is not affected in a mosaic egg chamber in which the two posterior PCs are wild type (GFP, clonal marker). (E) Nuclear Stat is completely lost when the two PCs are mutant for RalA. In (C–E), red denotes Fas3 staining and grey Stat staining. (F,G) The Unpaired (Upd) ligand (in red) forms a gradient centred at the poles in heterozygous egg chambers (PCs are marked by Fas3 in green). By contrast, Upd expression is strongly reduced when the two PCs are mutant for RalA (G). (H,I) When posterior PCs are mutant, the oocyte shows anterior–posterior polarization defects, as evidenced by the mislocalization of the Staufen protein (in red). Green indicates GFP clonal marker. GFP, green fluorescent protein.
Does the reduction of Upd lead to non-autonomous defects posteriorly? It was shown previously that the Jak/Stat pathway is essential for specifying posterior follicle cells, which then signal back to the oocyte for anterior–posterior polarization (Xi et al, 2003; Fig 4G–H′). When polarity is normal, the Staufen protein forms a posterior crescent in the oocyte (Fig 5H). In egg chambers containing posterior RalA mutant PCs, the localization of Staufen was not normal and it was found centrally in strongly affected oocytes (Fig 5I), similar to mutants that fail to reorganize the microtubules (van Eeden & St Johnston, 1999).
Among follicle cells, PCs have been shown to be important in patterning the egg chamber and in establishing BCs (Xi et al, 2003). In this study, we identified RalA as a new key regulator of PC fate. RalA is essential both cell autonomously for maintaining PC differentiation and cell non-autonomously for patterning terminal follicle cells through Jak/Stat signalling. However, RalA mutations do not reproduce the full range of Jak/Stat mutations (Silver & Montell, 2001; Beccari et al, 2002; Ghiglione et al, 2002; McGregor et al, 2002; Xi et al, 2003), consistent with the fact that some Upd is still produced by RalA mutant PCs (Fig 5G). For example, the follicle cell markers MA33 and dpp–lacZ, the expression of which in stretched cells is controlled by Jak/Stat signalling (Xi et al, 2003), are expressed normally when PCs are mutant for RalA (data not shown). Altogether, our data indicate that the function of RalA is essential for maintaining the PC fate and for ensuring high levels of Upd expression, which are required for patterning the most terminal follicle cells, including BCs and posterior follicle cells. The RalA phenotype suggests the existence of a maintenance signal taking place around stage 6–7—that is, following egg chamber proliferation phase—which would be necessary to complete egg chamber patterning by providing sustained Jak/Stat activation.
Previous studies have shown that Ral proteins interact with Sec5 (Moskalenko et al, 2002; Sugihara et al, 2002) and Exo84 (Moskalenko et al, 2003; Formstecher et al, 2005) to regulate the exocyst function during proliferation and tumorigenesis (Camonis & White, 2005; Vitale et al, 2005; Rosse et al, 2006). Our in vivo study suggests a role for RalA in cell differentiation and patterning, independent of secretion. Not only Upd but also several non-secreted PC markers lose expression following RalA loss of function, reminiscent of a more general differentiation phenotype. Contrary to what would be expected of a secretion phenotype, the Upd protein does not accumulate within PCs that are mutant for RalA (Fig 5G). The analysis of sec5 mutations in the follicle cells showed that this gene is required for the positioning of the oocyte and for follicle cell morphology (Murthy & Schwarz, 2004), two phenotypes that we never observed in RalA mutant egg chambers. Finally, expression in BCs of RNA-mediated interference against the sec5, sec6, sec8 or sec15 genes did not show any phenotype (data not shown).
Thus, instead of showing a ubiquitous activity, our data indicate a cell-type-specific function for RalA in PCs, independent of secretion. Controlling the differentiation of PCs, which have a central organizing role through Jak/Stat ligand production, might represent a way to monitor the number of invasive cells during both normal development and tumour cell invasion. Interestingly, in mouse M1 myeloid leukaemia cells, Stat3 can activate Ral by controlling the expression of its exchange factor (Senga et al, 2001). These data suggest a conserved functional link between Ral proteins and Stat activity and provide a basis for the maintenance of Jak/Stat activity in PCs through a positive feedback loop involving RalA and Stat.
Methods
Genetics. A description of genetic markers and chromosomes can be found at FlyBase (http://flybase.bio.indiana.edu). RalA has been identified through P-element mutagenesis using a tissue-targeted mosaic screen in ovaries (Ghiglione et al, 2002; this study). RalAh1 is a viable, hypomorphic allele generated by imprecise excision of the RalAPG89 P-element as per standard protocols. Reverse transcription–PCR (RT–PCR) on both PG69 and PG89 lines showed residual expression, suggesting that these lethal alleles correspond to strong hypomorphic alleles. Green fluorescent protein negatively marked homozygous follicle cell clones for RalA were induced using the FLP/FRT (flipase/flipase recognition target) system. The following Drosophila stocks have been used: RalAPG69 FRT19A/FM7, RalAPG89 FRT19A/FM7 and RalAPL56 FRT19A/FM7. The other stocks used in this study are Upd–Gal4, slbo–Gal4, CY2–Gal4, slbo–lacZ, E(Spl)–lacZ, A101–lacZ; PZ80–lacZ, UAS–RalA wt, UAS–RalAS25N and UAS–RalAG20V.
Antibodies, immunostaining and imaging. Immunostaining of egg chambers was performed as described previously (Ghiglione et al, 2002). The following primary antibodies were used: rabbit Dome (1:200), mouse Fas3 (1:100; 7G10, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, IA, USA), mouse Fas2 (1:100; 1D4, DSHB), mouse EYA (1:100, 10H6, DSHB), rabbit β-galactosidase (1:1,000, Cappel, Fountain Pkwy, OH, USA), rabbit Stat92E (1:500, a gift from S. Hou), rabbit human RALB (1:500, BD Transduction Laboratories, Franklin Lakes, NJ, USA), rabbit cleaved Casp3 (1:200, Cell Signalling), rabbit Upd (1:1,000, a gift from D. Harrison) and rabbit Staufen (1:5,000, a gift from D. St Johnston). Secondary antibodies were anti-rabbit Alexa 488 (1:400), CY5 (1:100) or Texas Red (1:100), anti-mouse Alexa 488 (1:400) or Texas Red (1:100) from Molecular Probes (Eugene, OR, USA).
Confocal images were taken using a Leica TCS-SP1 or a Zeiss LSM 510 META confocal microscope using × 25 and 0.80 NA and × 40 and 1.3 NA oil immersion objectives. Other images were taken using a Nikon Coolpix 990 digital camera and processed using Photoshop 7.0 (Adobe).
Immunoblot analysis. For preparation of ovarian extracts, ten ovaries were dissected in ice-cold PBS. The PBS was removed and replaced with Laemmli loading buffer. Ovaries were homogenized using a pestle and then centrifuged briefly to pellet debris. An equivalent of two ovaries per lane was loaded on a 12% SDS–polyacrylamide gel electrophoresis gel under reducing conditions and blotted onto nitrocellulose filter. The rabbit human RALB and the horseradish peroxidase-conjugated secondary antibodies (1:2,000, Vector, Berlingame, CA, USA) were used to detect RalA. The bands were visualized using enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
supplementary Information
Acknowledgments
We thank M. Balakireva, D. Harrison, M. Grammont, S. Hou, D. Montell, P. Rorth, K. Sawamoto, T. Sexton, D. St Johnston, the DSHB and the Bloomington Stock Center for providing us with fly stocks and reagents, and M. Balakireva and members of our laboratory for helpful discussions. This work was supported by grants from the Centre National de la Recherche Scientifique, Ministère, the Indo-French Centre for Promotion of Advanced Research, the Association pour la Recherche Contre le Cancer and the European Molecular Biology Organization Young Investigator Program.
Footnotes
The authors declare that they have no conflict of interest.
References
- Balakireva M et al. (2006) The Ral/exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in Drosophila melanogaster. Mol Cell Biol 26: 8953–8963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari S, Teixeira L, Rorth P (2002) The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech Dev 111: 115–123 [DOI] [PubMed] [Google Scholar]
- Besse F, Pret AM (2003) Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 130: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rørth P (2007) Two distinct modes of guidance signalling during collective migration of border cells. Nature 448: 362–365 [DOI] [PubMed] [Google Scholar]
- Bourbon HM et al. (2002) A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev 110: 71–83 [DOI] [PubMed] [Google Scholar]
- Camonis JH, White MA (2005) Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol 15: 327–332 [DOI] [PubMed] [Google Scholar]
- Devergne O, Ghiglione C, Noselli S (2007) The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci 120: 3457–3464 [DOI] [PubMed] [Google Scholar]
- Formstecher E et al. (2005) Protein interaction mapping: a Drosophila case study. Genome Res 15: 376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, Cerezo D, Noselli S (2002) The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 129: 5437–5447 [DOI] [PubMed] [Google Scholar]
- Grammont M, Irvine KD (2002) Organizer activity of the polar cells during Drosophila oogenesis. Development 129: 5131–5140 [DOI] [PubMed] [Google Scholar]
- McGregor JR, Xi R, Harrison DA (2002) JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development 129: 705–717 [DOI] [PubMed] [Google Scholar]
- Mirey G, Balakireva M, L'Hoste S, Rosse C, Voegeling S, Camonis J (2003) A Ral guanine exchange factor–Ral pathway is conserved in Drosophila melanogaster and sheds new light on the connectivity of the Ral, Ras, and Rap pathways. Mol Cell Biol 23: 1112–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ (2003) Border-cell migration: the race is on. Nat Rev Mol Cell Biol 4: 13–24 [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA (2002) The exocyst is a Ral effector complex. Nat Cell Biol 4: 66–72 [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA (2003) Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem 278: 51743–51748 [DOI] [PubMed] [Google Scholar]
- Murthy M, Schwarz TL (2004) The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development 131: 377–388 [DOI] [PubMed] [Google Scholar]
- Prasad M, Montell DJ (2007) Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell 12: 997–1005 [DOI] [PubMed] [Google Scholar]
- Rorth P (2002) Initiating and guiding migration: lessons from border cells. Trends Cell Biol 12: 325–331 [DOI] [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J (2006) RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol 26: 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Winge P, Koyama S, Hirota Y, Yamada C, Miyao S, Yoshikawa S, Jin MH, Kikuchi A, Okano H (1999) The Drosophila Ral GTPase regulates developmental cell shape changes through the Jun NH(2)-terminal kinase pathway. J Cell Biol 146: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senga T, Iwamoto T, Kitamura T, Miyake Y, Hamaguchi M (2001) JAK/STAT3-dependent activation of the RalGDS/Ral pathway in M1 mouse myeloid leukemia cells. J Biol Chem 276: 32678–32681 [DOI] [PubMed] [Google Scholar]
- Silver DL, Montell DJ (2001) Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell 107: 831–841 [DOI] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y (2002) The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol 4: 73–78 [DOI] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D (1999) The polarisation of the anterior–posterior and dorsal–ventral axes during Drosophila oogenesis. Curr Opin Genet Dev 9: 396–404 [DOI] [PubMed] [Google Scholar]
- Vitale N, Mawet J, Camonis J, Regazzi R, Bader MF, Chasserot-Golaz S (2005) The small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J Biol Chem 280: 29921–29928 [DOI] [PubMed] [Google Scholar]
- Xi R, McGregor JR, Harrison DA (2003) A gradient of JAK pathway activity patterns the anterior–posterior axis of the follicular epithelium. Dev Cell 4: 167–177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information