Abstract
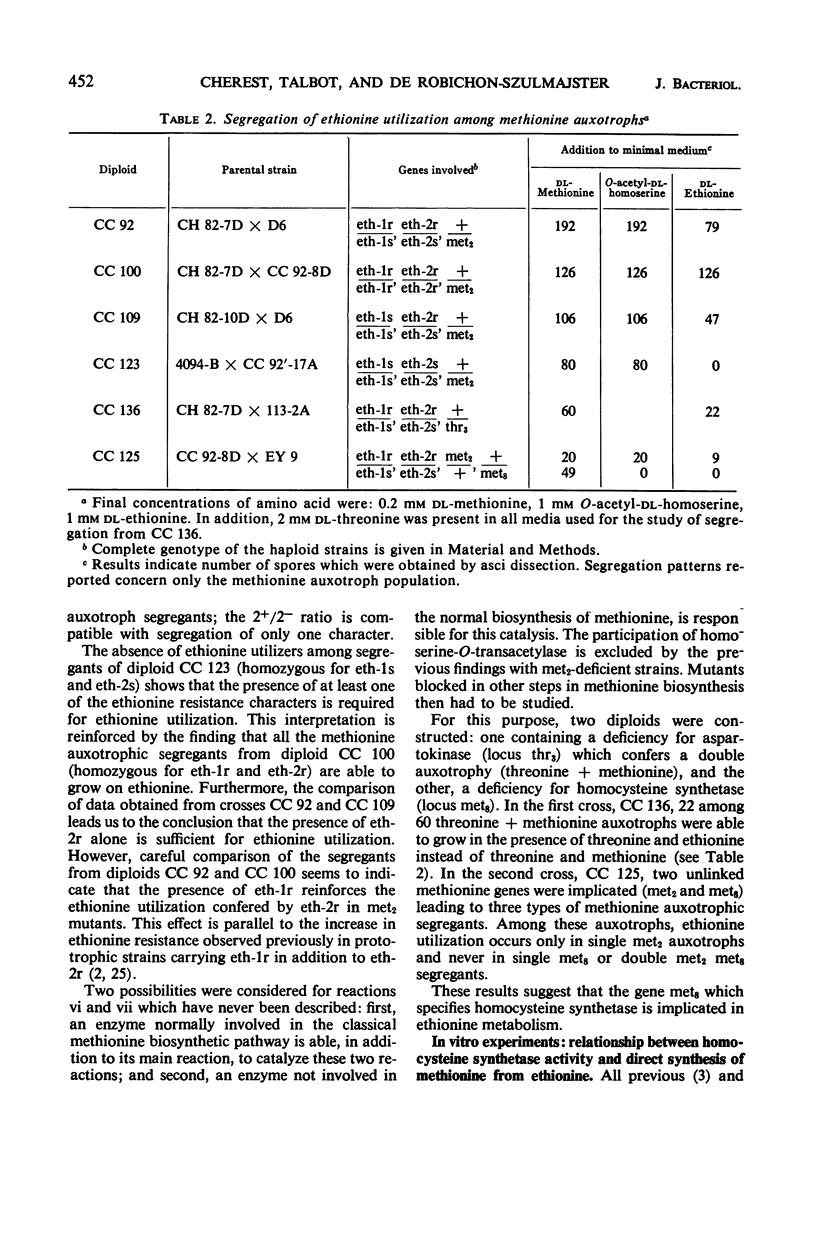
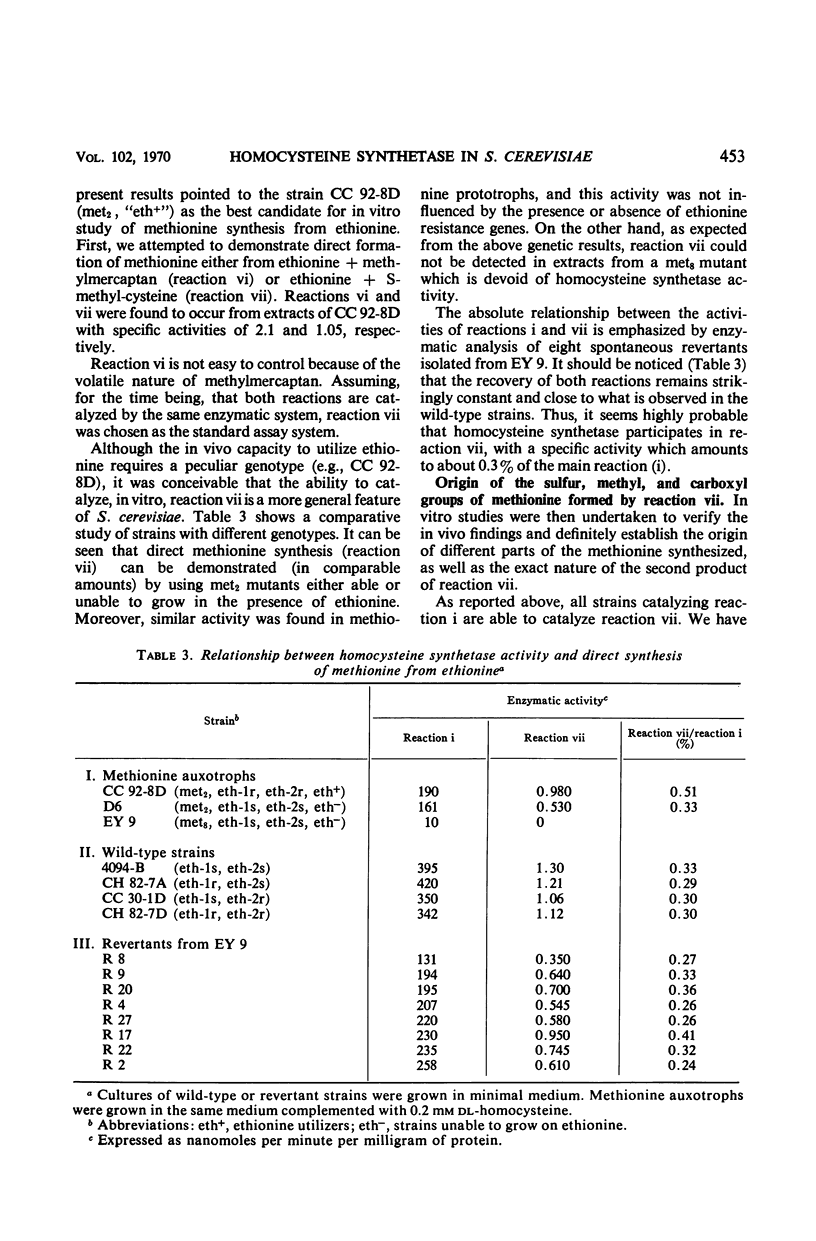
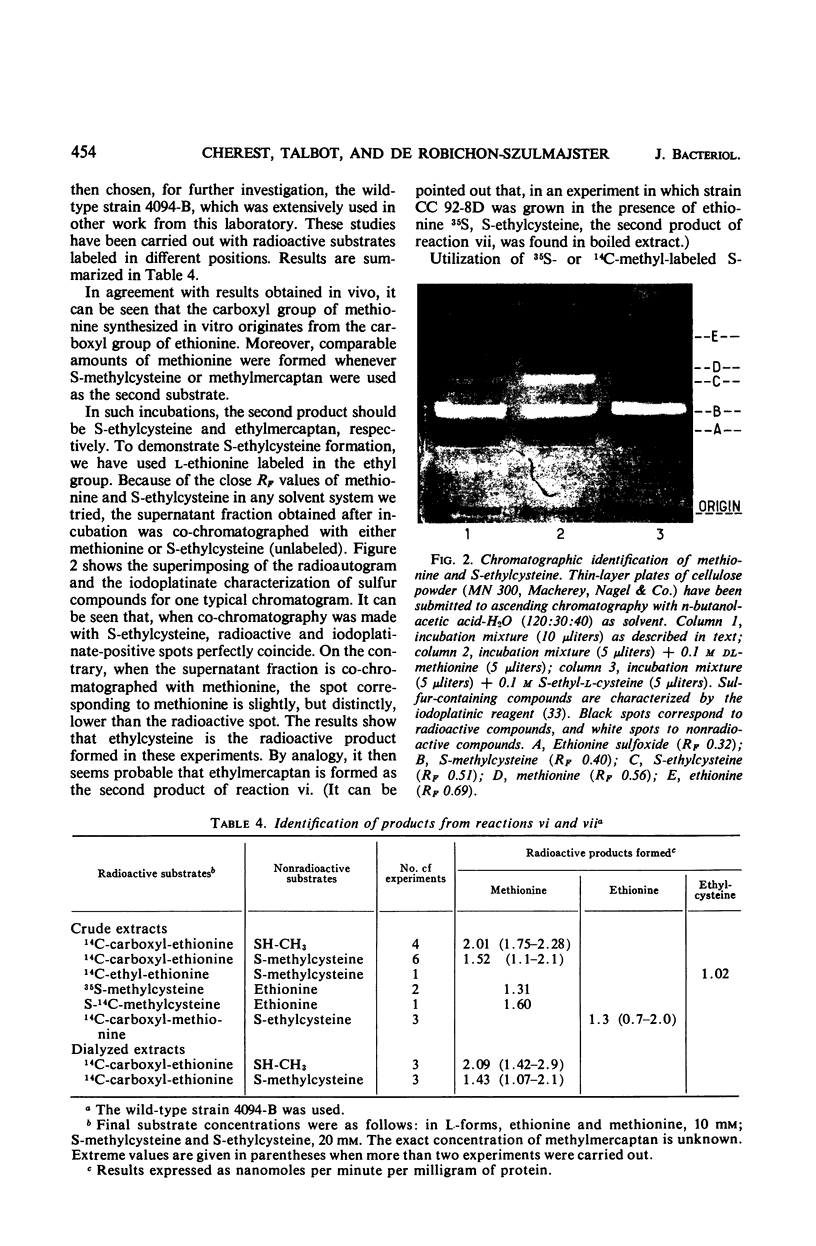
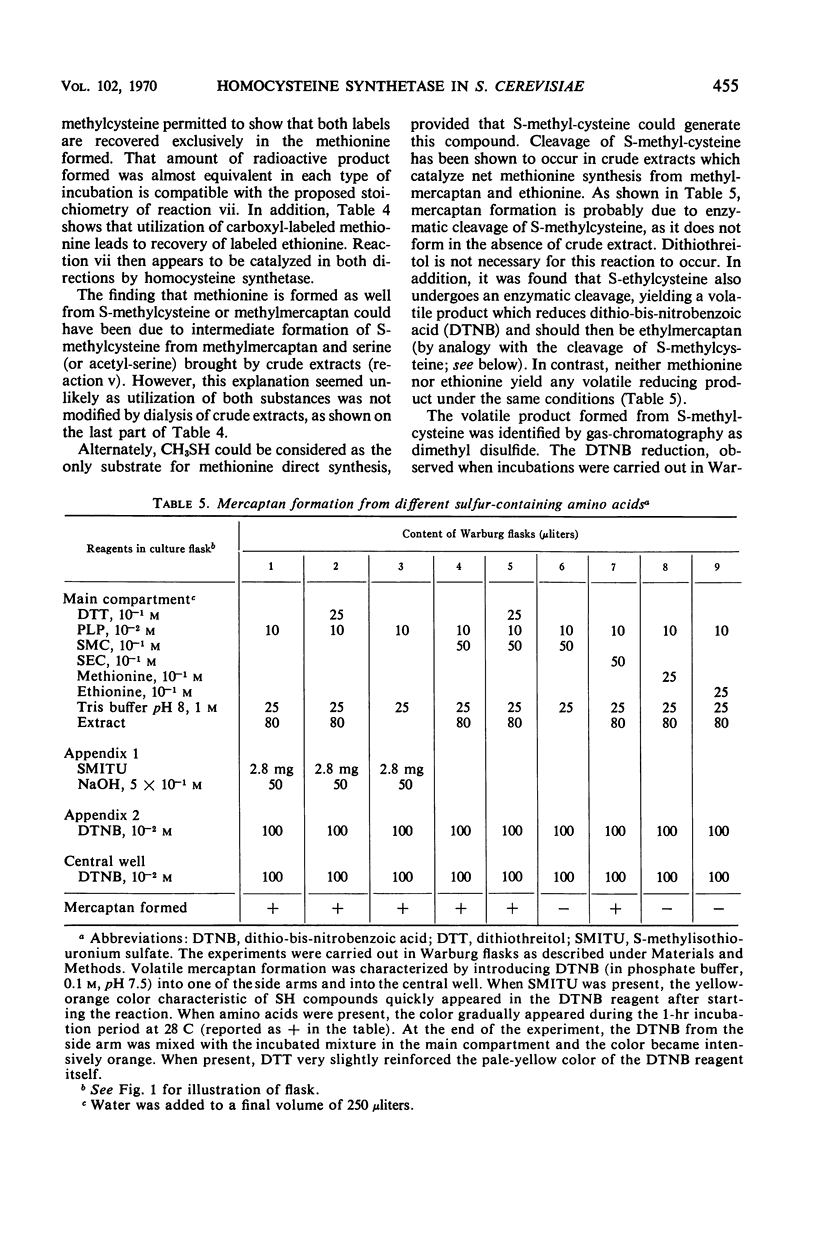
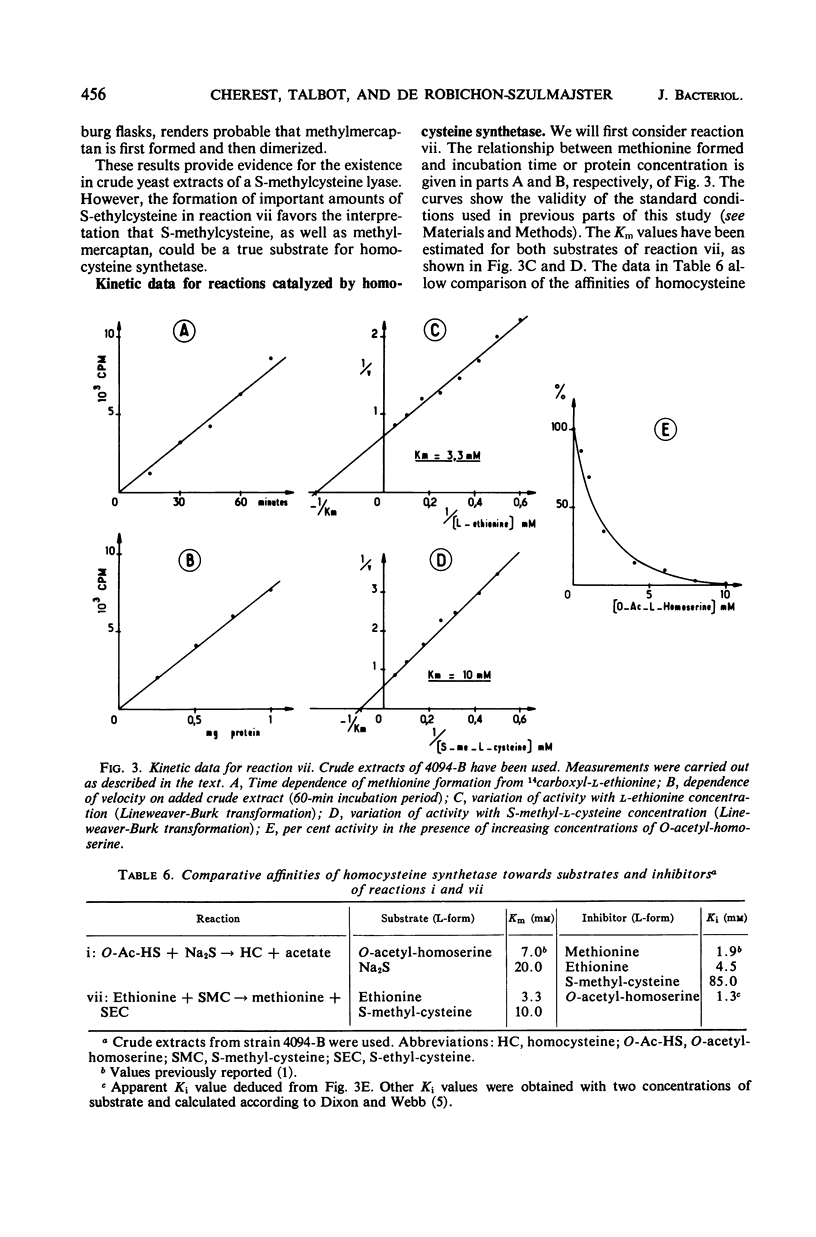
In vivo studies have shown that, in the absence of homoserine-O-transacetylase activity (locus met2), the C4-carbon moiety of ethionine is utilized (provided the ethionine resistance gene eth-2r is present) by methionine auxotrophs, except for met8 mutants (homocysteine synthetase-deficient). Concomitant utilization of sulfur and methyl group from methylmercaptan or S-methylcysteine has been demonstrated. In the absence of added methylated intermediates, the methyl group of methionine formed from ethionine is derived from serine. In vitro studies with crude extracts of Saccharomyces cerevisiae have demonstrated that this synthesis of methionine occurs by the following reactions: CH3-SH + ethionine ⇌ methionine + C2H5SH and S-methylcysteine + ethionine ⇌ methionine + S-ethylcysteine. In the forward direction, the second product of the second reaction was shown to be S-ethylcysteine; this reaction has also been found reversible, leading to ethionine formation. Genetic and kinetic data have shown that homocysteine synthetase catalyzes these two reactions, at 0.3% of the rate it catalyzes direct homocysteine synthesis: O-Ac-homoserine + Na2S → homocysteine + acetate. The three reactions are lost together in a met8 mutant and are recovered to the same extent in spontaneous prototrophic revertants from this strain. Methionine-mediated regulation of enzyme synthesis affects the three activities and is modified to the same extent by the presence of the recessive allele (eth-2r) of the regulatory gene eth-2. Affinities of the enzyme for substrates of both types of reactions are of the same order of magnitude. Moreover, ethionine, the substrate of the second reaction, inhibits the third reaction, whereas O-acetyl-homoserine, the substrate of the third reaction, inhibits the second reaction. An enzymatic cleavage of S-methylcysteine, leading to methylmercaptan production, has been shown to occur in crude yeast extracts. It is concluded that the enzyme homocysteine synthetase participates in the two alternate pathways leading to methionine biosynthesis in S. cerevisiae, one involving O-acetyl-homoserine and H2S, the other involving the 4-carbon chain of ethionine and a mercaptyl donor. Participation of the two types of reactions catalyzed by homocysteine synthetase, in in vivo methionine synthesis, has been shown to occur in a met2 partial revertant.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Robichon-Szulmajster H. Résistance a l'éthionine chez Saccharomyces cerevisiae. I. Etude génétique. Genetics. 1966 Oct;54(4):981–991. doi: 10.1093/genetics/54.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Talbot G., Robichon-Szulmajster H. Methionine biosynthesis from the 4-carbon skeleton of ethionine in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1968 Aug 21;32(4):723–730. doi: 10.1016/0006-291x(68)90299-4. [DOI] [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C., FLAVIN M. ENZYMATIC SYNTHESIS AND CLEAVAGE OF CYSTATHIONINE IN FUNGI AND BACTERIA. J Biol Chem. 1965 Jun;240:2537–2549. [PubMed] [Google Scholar]
- FLAVIN M., SLAUGHTER C. CYSTATHIONINE CLEAVAGE ENZYMES OF NEUROSPORA. J Biol Chem. 1964 Jul;239:2212–2219. [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine. Biochim Biophys Acta. 1967 Mar 15;132(2):400–405. doi: 10.1016/0005-2744(67)90158-1. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Enzymatic synthesis of cystathionine by extracts of spinach, requiring O-acetylhomoserine or O-succinylhomoserine. Biochem Biophys Res Commun. 1966 Nov 11;25(3):366–371. doi: 10.1016/0006-291x(66)90787-x. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Sulfuration of O-acetylhomoserine and O-acetylserine by two enzyme fractions from spinach. Biochem Biophys Res Commun. 1968 Apr 19;31(2):275–280. doi: 10.1016/0006-291x(68)90742-0. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Synthesis of homocysteine and cysteine by enzyme extracts of spinach. Biochem Biophys Res Commun. 1967 Apr 20;27(2):150–156. doi: 10.1016/s0006-291x(67)80054-8. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. Synthesis of cystathionine from O-acetylhomoserine in Neurospora: a step in methionine biosynthesis. Biochem Biophys Res Commun. 1968 Apr 5;31(1):124–130. doi: 10.1016/0006-291x(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Kerr D., Flavin M. Inhibition of cystathionine gamma-synthase by S-adenosylmethionine: A control mechanism for methionine synthesis in Neurospora. Biochim Biophys Acta. 1969 Feb 18;177(1):177–179. doi: 10.1016/0304-4165(69)90086-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAW G. A. Ability of S-methyl-L-cysteine to annul the inhibition of yeast growth by L-ethionine and by S-ethyl-L-cysteine. J Gen Microbiol. 1961 Jul;25:441–449. doi: 10.1099/00221287-25-3-441. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A., Metzenberg R. L. Positive control by the cys-3 locus in regulation of sulfur metabolism in Neurospora. J Mol Biol. 1968 Apr 28;33(2):423–437. doi: 10.1016/0022-2836(68)90199-x. [DOI] [PubMed] [Google Scholar]
- Maw G. A., Coyne C. M. The metabolism of S-methylcysteine in yeasts. Arch Biochem Biophys. 1968 Sep 20;127(1):241–251. doi: 10.1016/0003-9861(68)90222-1. [DOI] [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzenberg R. L., Parson J. W. Altered repression of some enzymes of sulfur utilization in a temperature-conditional lethal mutant of Neurospora. Proc Natl Acad Sci U S A. 1966 Mar;55(3):629–635. doi: 10.1073/pnas.55.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. P., Thompo J. F., Smith I. K. Utilization of S-methylcysteine and methylmercaptan by methionineless mutants of Neurospora and the pathway of their conversion to methionine. I. Growth studies. Biochim Biophys Acta. 1969 Jun 17;184(1):124–129. doi: 10.1016/0304-4165(69)90106-8. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine. An intermediate in the fungal biosynthesis of methionine. J Biol Chem. 1967 Sep 10;242(17):3884–3895. [PubMed] [Google Scholar]
- Phillips J. H., Kjellin-Stråby K. Studies on microbial ribonucleic acid. IV. Two mutants of Saccharomyces cerevisiae lacking N-2-dimethylguanine in soluble ribonucleic acid. J Mol Biol. 1967 Jun 28;26(3):509–518. doi: 10.1016/0022-2836(67)90318-x. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. O-SUCCINYLHOMOSERINE AS AN INTERMEDIATE IN THE SYNTHESIS OF CYSTATHIONINE BY ESCHERICHIA COLI. J Gen Microbiol. 1964 Sep;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Résistance a l'éthionine chez Saccharomyces cerevisiae. II. Etude physiologique. Genetics. 1966 Oct;54(4):993–1006. doi: 10.1093/genetics/54.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Magee P. T. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. I. Threonine deaminase. Eur J Biochem. 1968 Feb;3(4):492–501. doi: 10.1111/j.1432-1033.1967.tb19558.x. [DOI] [PubMed] [Google Scholar]
- Smith I. K., Thompson J. F. Utilization of S-methylcysteine and methylmercaptan by methionineless mutants of Neurospora and the pathway of their conversion to methionine. II. Enzyme studies. Biochim Biophys Acta. 1969 Jun 17;184(1):130–138. doi: 10.1016/0304-4165(69)90107-x. [DOI] [PubMed] [Google Scholar]
- Sorsoli W. A., Buettner M., Parks L. W. Cystathionine metabolism in methionine auxotrophic and wild-type strains of Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):1024–1029. doi: 10.1128/jb.95.3.1024-1029.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Moore D. P. Enzymatic synthesis of cysteine and S-methylcysteine in plant extracts. Biochem Biophys Res Commun. 1968 Apr 19;31(2):281–286. doi: 10.1016/0006-291x(68)90743-2. [DOI] [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]




