Abstract
Protease-activated receptor-2 (PAR2) is a 7-transmembrane G-protein-coupled tethered ligand receptor that is expressed by pancreatic acinar and ductal cells. It can be physiologically activated by trypsin. Previously reported studies (Namkung, W., Han, W., Luo, X., Muallem, S., Cho, K. H., Kim, K. H., and Lee, M. G. (2004) Gastroenterology 126, 1844–1859; Sharma, A., Tao, X., Gopal, A., Ligon, B., Andrade-Gordon, P., Steer, M. L., and Perides, G. (2005) Am. J. Physiol. 288, G388–G395) have shown that PAR2 activation exerts a protective effect on the experimental model of pancreatitis induced by supramaximal secretagogue (caerulein) stimulation. We now show that PAR2 exerts a worsening effect on a different model of experimental pancreatitis, i.e. one induced by retrograde pancreatic ductal infusion of bile salts. In vitro studies using freshly prepared pancreatic acini show that genetic deletion of PAR2 reduces bile salt-induced pathological calcium transients, acinar cell injury, and activation of c-Jun N-terminal kinase, whereas genetic deletion of PAR2 has the opposite or no effect on these pancreatitis-related events when they are elicited, in vitro, by caerulein stimulation. Studies employing a combination of trypsin inhibition and activation of PAR2 with the activating peptide SLIGRL show that all these differences indeed depend on the activation of PAR2. These studies are the first to report that a single perturbation can have model-specific and opposite effects on pancreatitis, and they underscore the importance of performing mechanistic pancreatitis studies using two dissimilar models of the disease to detect idiosyncratic, model-specific events. We suggest PAR2 activation exerts a worsening effect on the severity of clinical pancreatitis and that interventions interfering with PAR2 activation may be of benefit in the treatment of patients with severe pancreatitis.
Acute pancreatitis is a complex inflammatory disease of the pancreas that is of uncertain pathogenesis. No specific therapy for the disease has so far been identified, and as a result, treatment for this relatively common and, sometimes, lethal disease is limited to supportive care. Pancreatitis severity and disease morbidity/mortality are closely correlated leading to the widely held view that interventions that reduce the severity of an attack might have a favorable impact on clinical outcome. This concept has sparked considerable investigative interest directed at identifying factors and events that regulate pancreatitis severity in the belief that their identification would lead to therapies that might minimize disease severity. For the most part, studies pursuing that goal have employed experimental models of acute pancreatitis induced in laboratory animals, rather than clinically derived material, and the results obtained using those models have been assumed to reflect features that would be relevant to the clinical disease as well.
The two best characterized experimental models of acute pancreatitis are the secretagogue-induced (1) and the bile salt-induced (2) models. In the secretagogue-induced model, either rats or mice are given repeated supramaximally stimulating doses of the cholecystokinin analog caerulein, and they develop a highly reproducible but relatively mild and transient form of the disease, which reaches its maximal severity within 24 h of the start of caerulein administration. Although widely employed in mechanistic studies dealing with pancreatitis, the caerulein-induced model has been criticized by many who have questioned its clinical relevance because there is no evidence to suggest that clinical pancreatitis is triggered by supramaximal secretagogue stimulation.
Use of the bile salt-induced pancreatitis model eliminates this concern regarding clinical relevance because, in contrast to the caerulein model, the events that are believed to trigger clinical biliary or gallstone pancreatitis (i.e. pancreatic duct outflow obstruction and/or reflux of bile into the pancreatic duct) are indeed replicated during induction of this model (2). Bile salt-induced pancreatitis is induced by the low pressure retrograde perfusion of the pancreatic duct with a bile salt solution such as sodium taurocholate, and like the caerulein model, it reaches its maximal level of severity within 24 h of duct infusion. Use of the bile salt model has usually been confined to rats and larger animals because of technical reasons (i.e. the ease of controlled pancreatic duct infusion), and it has not been employed in studies using mice. In recently published studies, however, we have shown that the sodium taurocholate model can, in fact, be adapted for use in mice (3) and that the use of this model in genetically modified mouse strains can provide valuable insights into mechanistic issues related to pancreatitis (4). Virtually all of our observations characterizing this bile salt-induced pancreatitis model have been confirmed in a series of studies recently reported by Wittel et al. (5).
PAR2,2 one of the four members of the PAR family, is widely expressed outside of the pancreas, but it is also present within the pancreas, where it is expressed by a variety of cell types, including endothelial cells, nerve cells, cells of the immune system, duct cells, and acinar cells (6–8). PAR2 is subject to activation by trypsin as well as mast cell tryptase (9), and this, combined with the frequently made observation that intrapancreatic activation of trypsinogen occurs in both clinical and experimental pancreatitis, has suggested that pancreatic PAR2 might play an important role in pancreatitis. Indeed, recently reported studies by Namkung et al. (6) have shown that pharmacological activation of PAR2 can reduce the severity of caerulein-induced pancreatitis in rats, and in independent studies, we have shown that the severity of caerulein-induced pancreatitis is increased by genetic deletion of PAR2 in mice (7). Taken together, these two studies indicate that PAR2 exerts a protective effect on secretagogue-induced pancreatitis, and they led the authors to speculate that interventions designed to activate PAR2 might be of benefit in the treatment of patients with clinical acute pancreatitis. More recently, Singh et al. (10) have suggested that PAR2 activation in pancreatic acinar cells might protect against secretagogue-induced pancreatitis by promoting digestive enzyme secretion from those cells.
We wondered, however, if the effects of PAR2 activation on the secretagogue-induced model of pancreatitis might be idiosyncratic to that model and not generally applicable to pancreatitis. In this study, we report the results of studies that have further examined the role of PAR2 in pancreatitis using the alternative and, we believe, more clinically relevant bile salt-induced experimental model of pancreatitis. We show that, in contrast to our findings with the caerulein model, genetic deletion of PAR2 markedly reduces the severity of bile salt-induced pancreatitis. Furthermore, we show that genetic deletion of PAR2 interferes with the pathological effects of bile salts on pancreatic acini under in vitro conditions, whereas activation of PAR2 promotes those in vitro changes.
EXPERIMENTAL PROCEDURES
Experimental Animals—All experiments were performed using non-sex-selected wild type C57/Bl/6 mice (20–30 g) purchased from Charles River Laboratories (Wilmington, MA) and 20–30-g PAR2–/– mice, of either sex, that had been bred from founder C57/Bl/6 knock-out animals kindly donated by Dr. P. Andrade-Gordon (Drug Discovery, Johnson & Johnson Pharmaceutical Research and Development, Spring House, PA) (11). The animals were housed in temperature-controlled (23 ± 2 °C) rooms with a 12:12 h light:dark cycle, fed standard laboratory chow, and allowed water ad libitum. All experiments were performed according to protocols approved by the Animal Care and Use Committee of the Tufts Medical Center.
Reagents—SLIGRL-NH2 (PAR2-activating peptide), TFLLR-NH2 (PAR1-activating peptide), and LRGILS-NH2 (reverse PAR2-activating peptide) were synthesized by the Tufts University Core Facility (Boston, MA). The amylase substrate (2-chloro-p-nitrophenyl-α-maltotrioside), the lactate dehydrogenase substrate (lactate), and the indicator for lactate dehydrogenase assay (nicotinamide adenine dinucleotide) were purchased from Diagnostics Chemical Ltd. (Oxford, CT). Antibodies against JNK and phospho-JNK (p-JNK) were from Cell Signaling (Beverly, MA). Pluronic F-127 and Fura-2/AM were purchased from Molecular Probes (Eugene, OR). Dulbecco's modified Eagle's medium mixed 1:1 with Ham's salts was from Invitrogen. Nitex® mesh filters were purchased from Sefar America (Kansas City, MO). Choline- and methionine-deficient diet was obtained from Dyets Inc. (Bethlehem, PA). Caerulein, dl-ethionine, sodium taurocholate, taurolithocholic acid 3-sulfate disodium salt (TLCS), soybean trypsin inhibitor (SBTI), and all other chemicals were of analytical grade and purchased from Sigma.
Induction of Pancreatitis and Evaluation of Pancreatitis Severity—Bile salt-induced pancreatitis was elicited as described by Laukkarinen et al. (3) by retrograde pancreatic duct infusion using either 50 μl of 37 mm (2%) sodium taurocholate or 50 μl of 3 mm (0.18%) TLCS in phosphate-buffered saline at a rate of 5 μl/min. Secretagogue-induced pancreatitis was elicited by giving mice 12 hourly intraperitoneal injections of caerulein (50 μg/kg body weight per injection). Animals were sacrificed by CO2 asphyxiation 24 h after the start of caerulein administration. Pancreatitis severity in the bile salt and secretagogue models was evaluated at the time of sacrifice by quantitating hyperamylasemia, pancreatic edema (i.e. pancreatic water content), pancreatic inflammation (i.e. pancreatic myeloperoxidase activity), and acinar cell injury/necrosis as described previously (7). Randomly selected regions of the pancreas were used for measurements involving the gland in the caerulein-induced model as that model is characterized by changes that are diffusely distributed within the gland. On the other hand, pancreatic measurements in the bile salt models were all made using portions of the pancreatic head as those models are characterized by changes that are primarily, and most reproducibly, localized to the pancreatic head. For this purpose, the mouse pancreatic head was defined as the portion of the gland that is located within 5 mm of the lesser duodenal curvature.
Diet-induced pancreatitis was elicited by feeding female mice (13–15 g) a choline-deficient 0.5% ethionine-supplemented diet (3 g diet/24 h) for 72 h preceded by a 24-h period of fasting as described previously (12). They were then given regular diet ad libitum and observed for an additional 3 days. Pancreatitis severity in the diet-induced model was evaluated by quantitation of animal mortality occurring within 2 or 3 days of resuming the regular diet.
Preparation of Pancreatic Acini and Fragments for in Vitro Studies—Pancreatic acini (1–100 cells per cluster) were freshly prepared for each experiment using collagenase digestion as described previously (13). For studies involving amylase secretion and trypsinogen activation, the acini were filtered through a 150-μm Nitex® filter. Small pancreatic acini (1–10 cells per cluster) used to evaluate calcium transients or lactate dehydrogenase leakage were filtered through a 40-μm Nitex® filter. For preparation of pancreatic fragments, freshly harvested portions of pancreas were minced, in the absence of collagenase, to create small (<0.2-mm3) pieces as described previously (4).
Measurement of Calcium Transients within Single Acinar Cells—Freshly prepared small pancreatic acini (1–10 cells per cluster) were pre-loaded with 1 μm Fura-2/AM in the presence of 0.0005% Pluronic F-127 for 10 min at room temperature followed by extensive washing, and calcium transients in single cells within the acinar cluster were monitored using a Nikon Eclipse TEU 2000 inverted microscope as described previously (13). Interference filters on a rotational filter wheel allowed selection of alternate excitation wavelengths of 340 and 380 nm from a xenon light source. Images of fluorescence emission at 510 nm were collected and sent, via an intensified charge-couple device, to a dedicated digital image analysis system (IPLAB 3.6 with ratio plus, Scanalytics, Fairfax, VA). Background subtraction was carried out independently at each of the excitation wavelengths. The ratios of fluorescence emission (510 nm) at the two excitation wavelengths (340 and 380 nm) were determined and expressed as the ratio of 340/380 fluorescence. Soybean trypsin inhibitor (SBTI), when present, was added 30 min prior to addition of caerulein or sodium taurocholate. When present, 1 mm of the PAR2-activating peptide (SLIGRL), the reverse PAR2-activating peptide (LRGILS), or the PAR1-activating peptide (TFLLR) were also added at the same time as caerulein or sodium taurocholate. All peptides were dissolved in the same buffer in which the cells had been suspended (i.e. Dulbecco's modified Eagle's medium/F-12 containing sodium pyruvate and 0.1% bovine serum albumin) (4, 13).
Other Measurements—Cell injury, after exposure to sodium taurocholate, was quantitated by measuring the rate of LDH leakage over 20 min. LDH was measured by determining the rate of increase in absorption at 340 nm because of the conversion of NAD+ to NADH as l-lactate is converted to pyruvate. For this purpose, LDH leakage/20 min was expressed as a percent of total LDH content (measured after lysis with 0.5% Triton X-100). When present, 5 μm (0.1 mg/ml) SBTI was added 30 min prior to addition of caerulein or sodium taurocholate, and the 1 mm of the PAR-related peptides SLIGRL, LRGILS, and TFLLR was added coincident with caerulein or sodium taurocholate. Activation of JNK was evaluated, by immunoblot analysis, monitoring formation of p-JNK as described previously (4). For this purpose, cytoplasmic extracts were exposed to antibodies directed against p-JNK (Thr-183/Tyr-185), and p-JNK levels were expressed as a fraction of total JNK.
Analysis of Data—The results reported in this study are expressed as mean ± S.E. values obtained from four or more independent experiments (in vivo studies) or three or more independent experiments each analyzed in duplicate (in vitro studies). In all figures, the vertical bars denote S.E. values. Statistical evaluation of data was accomplished using Student's t test when two groups were compared and analysis of variance when multiple groups were compared. A p value of <0.05 was considered to indicate significant difference. The calcium results were analyzed using Wilcoxon rank-sum test. A p value of ≤0.0083, which is the Bonferroni adjusted p-value to control the experimentwise alpha at 0.05 when there are six comparisons, was considered to indicate significant difference.
RESULTS
Differential Effects of PAR2 Deletion on Bile Salt- and Secretagogue-induced Pancreatitis—Experimental pancreatitis was induced in wild type and PAR2–/– mice by either retrogradely infusing 50 μl of 2% sodium taurocholate into the pancreatic duct or by administering 12-h supramaximally stimulating doses (50 μg/kg) of caerulein. The animals were sacrificed 24 h after the start of pancreatitis induction, and the severity of pancreatic injury was evaluated by quantitating hyperamylasemia, pancreatic edema (i.e. pancreatic water content), pancreatic inflammation (i.e. pancreatic myeloperoxidase activity), and the extent of morphometrically detectable acinar cell injury/necrosis.
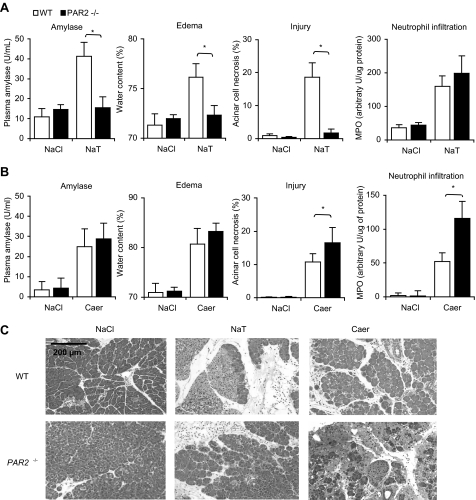
As shown in Fig. 1, the effects of PAR2 deletion on pancreatic injury during sodium taurocholate-induced and caerulein-induced pancreatitis were markedly different. In the bile salt-induced model, PAR2 deletion was associated with markedly reduced levels of hyperamylasemia, pancreatic edema, and acinar cell injury necrosis but similar levels of inflammation (Fig. 1, panels A and C). In contrast, in the secretagogue-induced model, PAR2 deletion was associated with a significant increase in the extent of acinar cell injury/necrosis and pancreatic inflammation but not in hyperamylasemia or edema (Fig. 1, panels B and C).
FIGURE 1.
Effects of PAR2 deletion on pancreatitis induced by either intraductal infusion of sodium taurocholate or supramaximal secretagogue stimulation. Pancreatitis was induced by either retrograde ductal infusion with NaCl alone, 2% sodium taurocholate (NaT)(panels A and C), or repeated administration of 50 μg/kg caerulein (caer)(panels B and C) as described in the text. MPO, myeloperoxidase. Mice were sacrificed 24 h after the start of pancreatitis induction, and pancreatitis severity was quantitated by measuring serum amylase activity, pancreatic water content (i.e. edema), acinar cell injury/necrosis, and pancreatic myeloperoxidase content (i.e. pancreatic inflammation). Results obtained from wild type mice (open columns) were compared with those obtained from PAR2–/– mice (closed columns). Results shown are mean ± S.E. values for 4–6 animals in each group. Asterisks denote p < 0.05 in bracketed groups. Panel C presents typical photomicrographs of hematoxylin and eosin-stained samples obtained from animals in each group. Note the reduction in edema and necrosis in the sample obtained from a PAR2–/– mouse subjected to sodium taurocholate-induced pancreatitis, but the increased pancreatic edema and necrosis in the sample obtained from a PAR2–/– mouse subjected to caerulein-induced pancreatitis when each of those samples were compared with comparable samples obtained from wild type (WT) control animals.
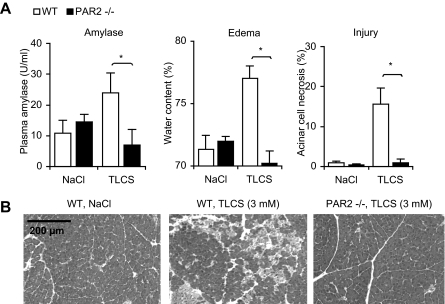
Similar studies were performed in which an alternative bile salt (TLCS) was infused into the pancreatic duct, and the results of those studies are shown in Fig. 2. As had been observed following infusion of sodium taurocholate, retrograde ductal infusion of TLCS (50 μl, 3 mm (i.e. 0.18%)) resulted in hyperamylasemia, pancreatic edema, and extensive acinar cell injury/necrosis, but PAR2 deletion markedly reduced the extent of each of these features of pancreatic injury. In contrast to these observations noted in the bile salt and secretagogue-induced models of pancreatitis, no change in the mortality rate of choline deficient diet-induced pancreatitis was associated with genetic deletion of PAR2 (see supplemental Fig. 1).
FIGURE 2.
Effect of PAR2 deletion on pancreatitis induced by intraductal infusion of TLCS. Pancreatitis was induced by either retrograde ductal infusion with NaCl alone or 3 mm (0.18%) TLCS. Mice were sacrificed 24 h after the start of pancreatitis induction, and pancreatitis severity was quantitated by measuring serum amylase activity, pancreatic water content (i.e. edema), and acinar cell injury/necrosis (panel A). Results obtained from wild type (WT) mice (open columns) were compared with those obtained from PAR2–/– mice (closed columns). Results shown are mean ± S.E. values for four animals in each group. Asterisks denote p < 0.05 in bracketed groups. Micrographs (panel B) are of hematoxylin and eosin-stained samples obtained from animals that received 3 mm TLCS. Note the reduction in edema and necrosis in the sample obtained from a PAR2–/– mouse subjected to TLCS-induced pancreatitis compared with the sample obtained from a wild type animal.
Taken together, our results indicate that the effects of PAR2 deletion on pancreatic injury during pancreatitis are highly dependent upon the model of pancreatitis that is being studied. PAR2 deletion reduces pancreatic injury in bile salt-induced pancreatitis, and this presumably indicates that PAR2 activation promotes pancreatic injury in wild type animals subjected to bile salt-induced pancreatitis. In contrast, PAR2 deletion increases pancreatic injury in secretagogue-induced pancreatitis, and this presumably indicates that PAR2 activation exerts a protective effect in wild type animals experiencing secretagogue-induced pancreatitis.
We also quantitated the effects of PAR2 deletion on pancreatic inflammation in the sodium taurocholate and caerulein-induced models by measuring pancreatic myeloperoxidase activity. The worsening of caerulein-induced pancreatic injury that was associated with PAR2 deletion was paralleled by a similar worsening of pancreatic inflammation in that model (Fig. 1, panel B). In contrast, the protection against sodium taurocholate-induced injury that was associated with PAR2 deletion was not paralleled by any change in pancreatic inflammation (Fig. 1, panel A). This latter observation is consistent with recently reported findings indicating that pancreatic injury and inflammation during pancreatitis may be separately regulated phenomena (4).
Our unexpected and surprising finding that PAR2 has diametrically contrasting effects on the severity of pancreatic injury in the two models suggested that PAR2 expression and activation might exert its opposite effects on injury severity by differentially effecting early and critical acinar cell events that ultimately lead to acinar cell injury during pancreatitis. To address this possibility, we embarked on a series of in vitro studies aimed at characterizing the effects of PAR2 expression and activation on some of those events as follows: (a) intra-acinar cell activation of trypsinogen; (b) generation of pathological intra-acinar cell calcium transients; (c) acinar cell injury; and (d) intra-acinar cell activation of the MAP kinase JNK.
Genetic Deletion of PAR2 Does Not Alter the Extent of Trypsinogen Activation That Is Observed When Pancreatic Acini Are Exposed to Either 0.2% Sodium Taurocholate or to a Supramaximally Stimulating Concentration of Caerulein—Time-dependent intra-acinar cell activation of trypsinogen has been observed previously to occur when acini are incubated, in vitro, with bile salts or supramaximally stimulating concentrations of caerulein (14, 15). We evaluated the role of PAR2 in this process by comparing the extent of bile salt-and caerulein-induced trypsinogen activation that was observed in acini prepared from wild type mice to that observed in acini prepared from PAR2–/– mice. As expected, incubation of wild type acini with either sodium taurocholate (0.2%) or caerulein (100 nm) led to the rapid appearance of trypsin activity, but the time course, as well as the magnitude, of this response was not different from that observed when acini prepared from PAR2–/– mice were similarly tested (supplemental Fig. 2). These observations indicate that the process of intra-acinar caerulein-induced and bile salt-induced activation of trypsinogen and the level of active trypsin reached as a result of that trypsinogen activation are not subject to regulation by PAR2.
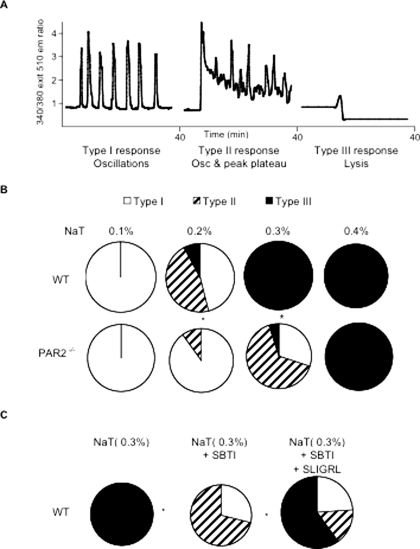
Differential Effects of PAR2 Deletion on Bile Salt- and Secretagogue-induced Acinar Cell Calcium Transients—Pancreatic acini obtained from wild type and PAR2–/– mice were loaded with Fura-2/AM, and calcium transients following exposure to varying concentrations of sodium taurocholate and caerulein were monitored in individual cells. Three response patterns to sodium taurocholate were identified as follows: (a) type I response was defined as one in which repeated oscillations of [Ca2+]i levels, superimposed upon a stable and flat resting free [Ca2+]i, were observed; (b) type II response was defined as one in which repeated Ca2+ oscillations were superimposed upon a peak plateau rise and fall of [Ca2+]]i; and (c) type III response was defined as one characterized by a sudden fall in Fura-2 fluorescence to a level below the initial base line (Fig. 3, panel A). Type I responses have, in general, been considered to be “physiological responses,” triggered when pancreatic acinar cells are exposed to secretory doses of secretagogues (13, 16–18), whereas type II responses are considered to be “pathological responses.” Type III responses are also pathological responses, but in this case, they are responses that would be expected to occur when acinar cells are lysed, and in our studies employing bile salts, this could be caused by the detergent effects of those bile salts.
FIGURE 3.
Effect of PAR2 deletion on calcium transients induced by exposure of isolated acini to sodium taurocholate. Panel A shows the three typical response patterns noted after exposure to sodium taurocholate (NaT), i.e. oscillations superimposed upon a stable and unchanged base line (type I), oscillations superimposed upon a peak-plateau rise and fall of the base line (type II), and a sudden and persistent decline of the base line (type III) after addition of the bile salt. The frequencies with which these responses were noted in individual acinar cells are shown in panels B and C. Panels B and C, open areas in the pie graphs depict the portion of cells with type I responses; hatched areas indicate the portion of cells with type II responses; and black areas depict the portion of cells with type III responses. Panel B, calcium responses between wild type (WT) and PAR2–/– acini are compared. Asterisks denote p < 0.0001 when results from wild type and PAR2–/– acini where compared. Panel C, pie graphs depict results obtained from wild type acini exposed to 0.3% sodium taurocholate alone, pre-exposed to 5 μm SBTI prior to sodium taurocholate (NaT), or pre-exposed to 5 μm SBTI followed by 1 mm SLIGRL and sodium taurocholate. The open areas depict the portion of cells with type I response; hatched areas indicate the portion of cells with type II response, and black areas indicate the portion of cells with type III response. Asterisks denote p < 0.0001 when acini exposed to sodium taurocholate plus SBTI were compared with the response from acini exposed only to sodium taurocholate, and when the response of acini exposed to sodium taurocholate plus SBTI plus SLIGRL were compared with those from acini exposed to sodium taurocholate plus SBTI. Results shown in panels B and C reflect findings noted by examining 20–25 cells in each of three independent experiments.
Calcium responses to varying concentrations of caerulein were noted to fall into two patterns. At maximally or submaximally stimulating secretory concentrations of caerulein, an oscillatory pattern was observed whereas supra-maximally stimulating concentrations of caerulein triggered a peak plateau response that lacked oscillations. Cell lysis, either noted microscopically or manifested by the loss of Fura-2 fluorescence, was not observed after exposure to even supra-maximally stimulating concentrations of caerulein.
As shown in Fig. 3, panel B, the response patterns in acinar cells exposed to sodium taurocholate were found to be dependent upon the expression of PAR2. More than 80% of acinar cells from wild type mice incubated with 0.2% sodium taurocholate manifested a type II or type III (i.e. pathological) response pattern, and higher concentrations of the bile salt elicited a type III or lysis response in virtually all of those cells. In contrast, 80% of acinar cells from PAR2–/– mice manifested a type I (i.e. physiological) response when exposed to 0.2% sodium taurocholate, and following exposure to 0.3% sodium taurocholate, only 10% of PAR2–/– acinar cells manifested a type III (i.e. lysis) response. With 0.3% sodium taurocholate, roughly 25% of cells manifested type I and 65% manifested type II responses (p < 0.0001 when compared with the responses obtained from wild type acini incubated with 0.2 and 0.3% sodium taurocholate, respectively, Wilcoxon rank-sum test). Similar studies were performed to examine the response patterns manifested by acinar cells obtained from wild type and PAR2–/– mice when those cells were exposed to either a maximally or a supramaximally stimulating concentration of caerulein. In each case, the maximally stimulating concentration of caerulein (0.1 nm) elicited a type I response in virtually all of the cells, and the supramaximally stimulating concentration of caerulein (1 nm) elicited a peak plateau response in virtually all of the cells regardless of whether those cells were obtained from wild type or PAR2–/– mice (supplemental Fig. 3). These observations with caerulein have been reported previously by our group (13).
These studies suggest that the expression of PAR2 by acinar cells makes those cells more sensitive to bile salt, but not secretagogue-induced, generation of pathological intracellular calcium transients. To determine whether the sensitization of wild type acinar cells to sodium taurocholate-induced pathological calcium transient generation was mediated by trypsin-induced PAR2 activation, we performed additional studies in which the effects of inhibiting extracellular trypsin activity and nonprototypically activating PAR2 were evaluated. As shown in Fig. 3, panel C, the effects on pathological calcium transient generation of PAR2 deletion were recapitulated in acini from wild type mice when the extracellular trypsin inhibitor SBTI was present (p = 0.6522 Wilcoxon rank-sum test). On the other hand, that phenomenon was reversed by addition of the PAR2-activating peptide SLIGRL (p < 0.0001 Wilcoxon rank-sum test). In other studies (supplemental Fig. 4), we found that this effect of SLIGRL addition on pathological calcium transient generation was not observed when the reverse (i.e. inactive) PAR2 peptide LRGILS or the PAR1-activating peptide TLFFR was used in place of SLIGRL (supplemental Fig. 4). Taken together, these studies indicate that the presence and physiological activation of PAR2 on acinar cells sensitize those cells to bile salt-induced but not secretagogue-induced generation of pathological transients and that, in wild type acini, bile salt-induced activation of trypsinogen plays a critical role in this sensitization phenomenon.
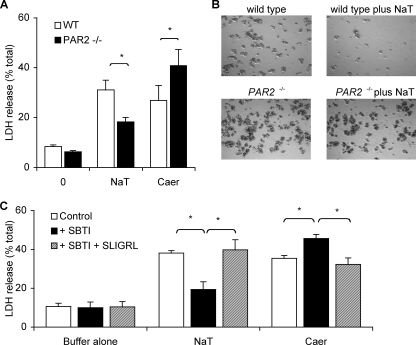
Differential Effects of PAR2 Deletion on Bile Salt- and Secretagogue-induced Acinar Cell Injury—The leakage of LDH from within acinar cells into the suspending medium is a widely used measure of cell injury or disruption. We evaluated the effects of sodium taurocholate and caerulein on this phenomenon in small acini obtained from wild type and PAR2–/– mice. These acini were prepared as were the ones we used for the calcium measurements, i.e. by filtration through a 40-μm filter, and they consisted of 1–10 acinar cells. They are more susceptible to cell death by supramaximal stimulation with caerulein or sodium taurocholate when compared with the acini prepared by filtration through a 150-μm filter (10–100 cells/per cluster) (data not shown). As shown in Fig. 4, panel A, the bile salt and the secretagogue both accelerate LDH leakage from wild type acinar cells. More importantly, however, we found that PAR2 deletion has diametrically opposite effects on bile salt and secretagogue-induced LDH leakage, i.e. it markedly reduces bile salt-induced leakage but it enhances secretagogue-induced LDH leakage. Furthermore, as shown by the phase contrast micrographs in Fig. 4, panel B, PAR2–/– acini are protected against the extensive lysis that is triggered by exposure to 0.3% sodium taurocholate. As shown in Fig. 4, panel C, the effects of PAR2 deletion on both bile salt- and secretagogue-induced cell injury/leakage could be replicated by addition of SBTI to the medium suspending wild type acini, and addition of the PAR2-activating peptide SLIGRL reversed this effect of SBTI. Thus, these observations indicate that the physiological expression and activation of PAR2 on acinar cells promote bile salt-induced cell injury/lysis, although it protects acinar cells from secretagogue-induced cell injury/lysis. Similar to what was observed when the [Ca2+]i changes were evaluated, we found that the reverse PAR2-activating peptide LRGILS and the PAR1-activating peptide TFLLR were unable to reverse the SBTI effect (supplemental Fig. 5). Taken together, these studies with SBTI and SLIGRL indicate that the cell injurious effects of sodium taurocholate and supramaximal stimulation with caerulein are, at least in part, regulated by activation of PAR2 and that in both cases PAR2 is activated by proteolytic activity (presumably tryptic) that is generated by either the bile salt or the secretagogue. In the former case, PAR2 activation increases acinar cell injury and in the latter case, PAR2 activation lessens cell injury.
FIGURE 4.
Effects of PAR2 deletion on LDH leakage induced by exposure of isolated small pancreatic acini to sodium taurocholate or caerulein. Panel A shows LDH leakage over 20 min when freshly prepared wild type (WT)(open columns) acini or PAR2–/– (closed columns) acini were exposed to buffer alone, 0.3% sodium taurocholate (NaT), or 100 nm caerulein (Caer). Panel B shows representative images from phase contrast microscopy performed 30 min after acini from wild type or PAR2–/– animals exposed to buffer alone or to 0.3% sodium taurocholate (NaT). Note loss of visible acini when wild type but not PAR2–/– acini were exposed to the bile salt. Panel C depicts results obtained when wild type acini were exposed to buffer alone, sodium taurocholate (0.3%), or caerulein (100 nm). Open columns depict results noted with no other additions; closed columns depict results obtained when acini were pre-exposed to 5 μm SBTI, and hatched columns depict results obtained when acini were pre-exposed to 5 μm SBTI followed by SLIGRL. Results shown reflect mean values obtained in >3 independent experiments. Vertical bars denote ± S.E., and asterisks denote p < 0.05 for bracketed groups.
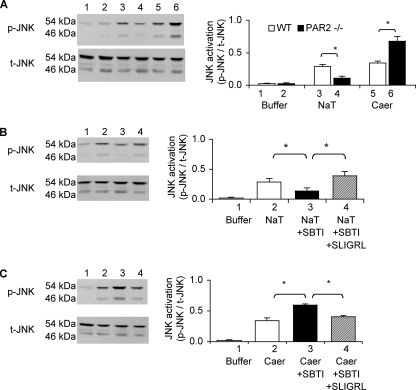
Differential Effects of PAR2 Deletion on Bile Salt- and Secretagogue-induced Activation of Acinar Cell JNK—As shown in Fig. 5, panel A, both sodium taurocholate and caerulein trigger the phosphorylation (i.e. activation) of the MAP kinase JNK in acinar cells. We found, however, that genetic deletion of PAR2 in acinar cells has opposite effects on bile salt- and secretagogue-induced JNK activation, i.e. it reduces the extent of bile salt-induced JNK activation whereas it increases the extent of caerulein-induced JNK activation. Furthermore, as shown in Fig. 5, panels B and C, sodium taurocholate-induced JNK activation in wild type acini is reduced by addition of SBTI and increased by addition of SLIGRL, whereas secretagogue-induced JNK activation responds conversely to these manipulations, i.e. it is increased in the presence of SBTI, and this increase is prevented by the presence of SLIGRL. Therefore, these observations replicate the pattern of response observed when the effects of sodium taurocholate and caerulein on cell injury/lysis were being monitored, i.e. PAR2 expression and activation (presumably by bile salt- or caerulein-triggered trypsinogen activation) promote bile salt-induced JNK activation, although it reduces secretagogue-induced JNK activation.
FIGURE 5.
Effects of PAR2 deletion on JNK activation induced by exposure of isolated pancreatic fragments to sodium taurocholate or caerulein. Panel A shows representative immunoblot for p-JNK and total JNK in pancreatic fragments exposed to buffer alone (lanes 1 and 2), sodium taurocholate (NaT)(lanes 3 and 4), or caerulein (Caer)(lanes 5 and 6). Lanes 1, 3, and 5 depict results noted in wild type (WT) fragments, and those in lanes 2, 4, and 6 reflect findings with PAR2–/– fragments. Plot at right shows densitometric quantitation of results obtained in three independent experiments performed in duplicate. JNK activation is expressed as the ratio of p-JNK to total JNK. Open columns represent wild type, and closed columns depict results from PAR2–/– pancreatic fragments. Panel B shows representative immunoblot for p-JNK and total JNK in wild type acini exposed to buffer alone (lane 1), buffer with 0.3% sodium taurocholate (lane 2), pre-exposed to 5 μm SBTI for 30 min and then exposed to sodium taurocholate (lane 3), and pre-exposed to 5 μm SBTI for 30 min and then exposed to sodium taurocholate plus SLIGRL (lane 4). Panel C shows immunoblot results obtained in similar experiments that were performed using 100 nm caerulein instead of sodium taurocholate. The conditions and lane assignments were otherwise identical to those in panel B. The plots to the right in panels B and C show densitometric quantitation of results obtained in three independent experiments performed in duplicate. Open columns represent results from acini exposed to buffer alone, and closed columns show results from fragments exposed to only sodium taurocholate or caerulein; right-hatched columns show results from fragments pre-exposed to SBTI and then exposed to sodium taurocholate or caerulein, and left-hatched columns show results from fragments pre-exposed to both SBTI and SLIGRL and then exposed to sodium taurocholate or caerulein. In all cases, results shown are mean values, and vertical bars show ± S.E. Asterisks denote p < 0.05 in bracketed groups.
DISCUSSION
In previously published reports, both Namkung et al. (6) and our group (7) have shown that the severity of pancreatic injury in caerulein-induced pancreatitis is reduced in rats or mice that have been pretreated with the PAR2-activating peptide SLIGRL. Our own study also showed that the severity of secretagogue-induced mouse pancreatitis is increased in PAR2–/– mice. This latter observation is also confirmed by the results of additional, but similar, studies that were performed as part of the currently reported project. Together, these various findings are all consistent with the conclusion that activation of PAR2 protects the pancreas from injury in pancreatitis and, indeed, that was the conclusion originally reached by both Namkung et al. (6) and our group. If valid, that conclusion could prompt efforts to reduce the severity of pancreatic injury in clinical pancreatitis by interventions designed to promote PAR2 activation. In this regard, however, note should also be made of several other observations reported in the paper published by Namkung et al. (6) that indicated that PAR2 activation during pancreatitis might also lead to a worsening of the disease (6). Those workers noted that systemic activation of PAR2 triggers or augments events that contribute to the multiple organ dysfunction syndrome and the systemic immune response syndrome. Thus, even if therapeutic activation of PAR2 did reduce the severity of pancreatic injury in clinical pancreatitis, that beneficial effect might very well be countered by a worsening of pancreatitis-induced systemic phenomena such as multiple organ dysfunction syndrome and systemic immune response syndrome.
We now show that the relationship between PAR2 activation and pancreatic injury during pancreatitis is even more complex than anticipated by these previous studies. As shown in this study, the severity of pancreatic injury during experimental pancreatitis can be either up-regulated or down-regulated by the expression and activation of PAR2. Indeed, the type of response observed depends upon the experimental method being used to induce pancreatitis. Although PAR2 deletion is associated with an increase in the severity of secretagogue-induced pancreatic injury, it is also associated with a decrease in the severity of bile salt-induced pancreatic injury. This phenomenon is not limited to the sodium taurocholate bile salt-induced model of pancreatitis because it is also observed when a different bile salt (i.e. TLCS) is used to elicit pancreatitis.
We are unaware of any previously published report showing that a single perturbation (in this case, genetic deletion of PAR2) can have such contrasting, model-specific effects on the severity of pancreatic injury in acute pancreatitis, and in our subsequent studies, we sought to clarify the mechanisms responsible for this phenomenon by identifying the pancreatitis-related acinar cell events that might be involved. At the outset, we recognized the need to pursue our search for relevant events by performing in vitro rather than in vivo studies because of the following: (a) our need to manipulate the state of PAR2 activation using the PAR2-activating peptide SLIGRL, and (b) the observation, previously reported (6), that the nontargeted in vivo activation of PAR2, elicited by systemic administration of SLIGRL, triggers an array of nonpancreatitis-specific events (e.g. hypotension, tachycardia, and capillary leak, etc.) that could markedly, but indirectly, affect early pancreatitis-related events in the pancreas.
Initially, we attempted to expand our studies using the mouse model of diet-induced pancreatitis, but we found that mortality from pancreatitis, the conventional measure of pancreatitis severity in the diet-induced model, was not altered by genetic deletion of PAR2 (supplemental Fig. 1). We also attempted to adapt the rat arginine-induced model of pancreatitis for use in mice, but in our hands, arginine-induced mouse pancreatitis that was of highly variable severity even within groups of animals simultaneously given arginine and, in mice, near lethal doses of arginine were needed to trigger pancreatitis. Finally, we considered performing studies using the rat model of pancreatitis elicited by infusing bile salts into the pancreatic duct and exploring the role of PAR2 activation in that model by systemically administering the PAR2-activating peptide SLIGRL. We rejected that approach because of previous reports that indicated systemic administration of SLIGRL could trigger profound hypotension and bradycardia, presumably as a result of the pharmacologic activation of PAR2 on cardiac, vascular endothelial, and vascular smooth muscle cells. We were concerned that those responses could themselves exert modifying effects on the severity of pancreatitis.
Because our in vitro studies aimed to explore mechanisms responsible for the fact that PAR2 deletion diminishes the severity of bile salt-induced pancreatitis, although it increases the severity of caerulein-induced pancreatitis, we reasoned that relevant events under in vitro conditions would have the following characteristics. (a) When compared with acini prepared from wild type acini, the relevant events would be down-regulated in PAR2–/– acini exposed to sodium taurocholate but up-regulated in PAR2–/– acini exposed to a supramaximally stimulating concentration of caerulein. (b) The relevant events would be reduced by addition of the trypsin inhibitor SBTI to wild type acini exposed to sodium taurocholate but increased by addition of SBTI to wild type acini exposed to a supramaximally stimulating concentration of caerulein. (c) The relevant events would be increased by addition of the PAR2-activating peptide SLIGRL to wild type acini exposed to sodium taurocholate but decreased by addition of SLIGRL to wild type acini supramaximally stimulated with caerulein. The physiological activator for PAR2 is trypsin, and during pancreatitis, PAR2 is presumably activated by trypsin that has become inappropriately activated within acinar cells and then either secreted or leaked into the interstitium of the gland. This led us to further reason that events related to the regulation of pancreatitis severity by PAR2 would, under in vitro conditions, be similarly altered by either genetic deletion of PAR2 or, in wild type acini, by SBTI-induced inhibition of extracellular trypsin and that those events would also be altered, but now in the opposite fashion, by exposure of wild type acini to the PAR2-activating peptide SLIGRL.
We elected to examine four of the acinar cell events that have been considered to play important roles in the early phases of acute pancreatitis: (a) generation of pathological calcium transients within acinar cells; (b) intra-acinar cell activation of trypsinogen; (c) acinar cell injury; and (d) activation of the MAP kinase JNK within acinar cells. We noted that genetic deletion of PAR2–/– in acini or, alternatively, addition of SBTI to wild type acini diminished the generation of pathological calcium transients in response to sodium taurocholate and that exposure of wild type acini to SLIGRL, even in the presence of SBTI, restored the generation of those bile salt-elicited pathological calcium transients. On the other hand, we found that secretagogue-induced generation of pathological calcium transients was not altered by any of these manipulations. Taken together, these observations lead us to suggest that the up-regulation of pathological calcium transient generation may play an important role in the PAR2-related worsening of bile salt-induced pancreatitis, but PAR2-related protection against secretagogue-induced pancreatitis is not the result of decreased generation of pathological calcium transients. Furthermore, our studies suggest that, in wild type acini, PAR2 regulates bile salt-triggered pathological calcium transient generation by responding to activated trypsin that is formed in response to addition of the bile salt but that this response can also occur when PAR2 is nonproteolytically activated by addition of SLIGRL.
Bile salts, at relatively high concentrations that depend upon the bile salt in question, can have detergent-like effects on acinar cells, and our own studies indicate that, with sodium taurocholate, that phenomenon is observed when wild type acini are exposed to sodium taurocholate concentrations of 0.3% or higher (6, 15). Expression and activation of PAR2 appear to sensitize acinar cells to the lysis that is observed in the presence of these high sodium taurocholate concentrations, i.e. PAR2 deletion or inhibition of trypsin-induced PAR2 activation by addition of SBTI reduces the frequency of type III (i.e. lytic) responses noted in acini incubated with 0.3% sodium taurocholate, and addition of SLIGRL to wild type acini increases the frequency of those responses. The mechanisms responsible for these effects of PAR2 activation remain to be determined.
At lower concentrations of sodium taurocholate, in the range of 0.2%, the bile salt triggers oscillatory changes in [Ca2+]i as well as oscillatory changes superimposed upon a peak/plateau rise and fall in [Ca2+]i. Others have shown that sub-detergent concentrations of bile salts trigger transporter-mediated active uptake of bile salts by pancreatic acinar cells and that bile salt uptake leads to inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase pump, depletion of calcium storage pools in the endoplasmic reticulum, activation of capacitative calcium influx, and a rise in [Ca2+]i (19). The studies reported here indicate that PAR2 activation plays an important role in this process, but they do not allow us to define the step(s) in this sequence that are altered by either PAR2 activation or PAR2 deletion.
It is generally believed that activation of trypsinogen during pancreatitis occurs subsequent to the triggering of altered acinar cell calcium dynamics and that intra-acinar cell activation of trypsinogen may be mediated by those pathological calcium transients that are generated during this very early phase of pancreatitis (20). In our studies, we confirmed previously made observations indicating that exposure of isolated pancreatic acini to either bile salts or to a supramaximally stimulating concentration of caerulein triggers trypsinogen activation (15, 21, 22). However, in our studies we also found that the extent of trypsinogen activation following exposure to sodium taurocholate or a supramaximally stimulating dose of caerulein was neither up- nor down-regulated by genetic deletion of PAR2. Based on this latter observation, we believe that it is unlikely that an alteration in the extent of trypsinogen activation is the basis for the contrasting effects of PAR2 deletion on the bile salt-induced and the secretagogue-induced models of pancreatitis.
Acinar cell injury and the activation of pro-inflammatory pathways are believed to be the acinar cell events that temporally follow intracellular trypsinogen activation during the evolution of pancreatitis (23). We elected to monitor cell injury by quantitating the leakage of LDH from acinar cells and the activation of pro-inflammatory pathways by monitoring activation of the MAP kinase JNK. For studies evaluating LDH leakage, we chose to use the “small acini” preparation that had been used for our single-cell calcium studies, and this may explain the high rate of LDH leakage that we observed in response to sodium taurocholate. For studies evaluating JNK activation, we chose to use tissue fragments rather than isolated acini because exposure to collagenase during the preparation of acini was noted to elevate the resting levels of p-JNK and, thus, interfere with quantitation of either bile salt or secretagogue-induced JNK activation.
Both LDH leakage and JNK activation occur, in vitro, when wild type acini are exposed to either sodium taurocholate or a supramaximally stimulating concentration of caerulein, and the magnitude of both events is altered by deletion of PAR2. Both LDH leakage and JNK activation in response to sodium taurocholate are reduced by PAR2 deletion, whereas both responses to supramaximal secretagogue stimulation are increased by deletion of PAR2. Furthermore, the activation of JNK and leakage of LDH in wild type acini exposed to the bile salt are both reduced by the presence of SBTI and increased by addition of SLIGRL, whereas the opposite occurs when wild type acini are exposed to caerulein in the presence of SBTI and SLIGRL. This constellation of responses leads us to conclude that PAR2 expression and activation by trypsin or SLIGRL play a critical role in the worsening of bile salt-induced pancreatitis by promoting both cell injury and the activation of JNK. Similarly, this constellation of responses leads us to conclude that PAR2 expression and activation play a critical role in reducing the severity of caerulein-induced pancreatitis by reducing the extent of cell injury and JNK activation.
Taken together, the findings reported here suggest that PAR2 activation plays a different role in the two models of pancreatitis. In the bile salt-induced model, PAR2 activation triggers the increased generation of pathological calcium transients, and this response leads to a worsening of pancreatic injury via a series of downstream events that include acinar cell injury and JNK activation. In the secretagogue-induced model, PAR2 activation reduces the severity of pancreatic injury by a mechanism that involves down-regulation of cell injury and JNK activation, but one that does not involve an alteration in the generation of pathological calcium transients. Pancreatic acinar and duct cells both express PAR2, and in both cell types, PAR2 stimulation accelerates exocrine secretion. Singh et al. (10) have recently suggested that PAR2 activation might reduce secretagogue-induced pancreatitis severity via its ability to stimulate the secretion of activated digestive zymogens from acinar cells. The validity of this hypothesis remains to be verified but, even if it is shown to be correct, it and the model proposed in the this study are not mutually exclusive. Indeed, PAR2 activation may reduce the severity of caerulein-induced pancreatitis by both stimulating exocrine secretion and by down-regulating both acinar cell injury and JNK activation.
Our current understanding of the mechanisms underlying acute pancreatitis is, to a great extent, based on the results of studies employing experimental models of the disease induced in laboratory animals. Most of those previous studies were performed using only a single model of the disease and, as shown here, that approach leaves open the possibility that the observed phenomena may have been model-specific, rather than general, features of pancreatitis. Thus, although such studies may elucidate critical mechanisms responsible for a particular model, it may be unsafe to conclude that those same mechanisms are operative in clinical pancreatitis.
To a great extent, this concern can be eliminated if similar results are observed in studies employing two or more dissimilar models because, in that case, the observed phenomena are likely to be relevant to pancreatitis in general and operative in the clinical disease as well. On the other hand, when differing responses are observed in dissimilar models, interpretation of those responses may be more difficult. In such cases and in the absence of clinical studies, we believe that the clinical relevance of the method of induction of a model should guide interpretation of the results.
Most patients with an identified cause for an attack of acute pancreatitis have biliary pancreatitis, and biliary pancreatitis is known to be triggered by the passage of biliary stones, sludge, or crystals through the terminal bilio-pancreatic duct (24). There is no evidence that the passage of stones or crystals can trigger release of supra-maximally stimulating amounts of secretagogues such as cholecystokinin. Rather, most observers believe that passage of stones or crystals triggers pancreatitis either by transiently causing pancreatic duct outflow obstruction or by promoting the reflux of bile into the pancreatic duct (25). Since the publications by Opie in 1901 (26, 27), there has been considerable controversy surrounding the question of whether biliary pancreatitis is triggered by bile reflux into the pancreatic duct or by transient pancreatic duct hypertension caused by outflow obstruction. The arguments for and against each of these hypotheses are multiple and beyond the scope of this study. However, during the course of eliciting bile salt-induced pancreatitis in mice, both bile reflux and ductal hypertension occur. Thus, forced to choose between results obtained with the bile salt-induced model and those obtained with secretagogue-induced model, we believe that results obtained with the former model are those that are most likely to be clinically relevant. Therefore, as a result, we are tempted to speculate that PAR2 activation promotes the worsening of clinical pancreatitis, and this leads us to suggest that interventions designed to interfere with either PAR2 activation or the downstream responses to that activation would favorably affect the outcome of clinical acute pancreatitis.
Supplementary Material
Acknowledgments
We are grateful to Dr. P. Andrade-Gordon, Johnson & Johnson Pharmaceutical Research and Development, for generously providing us with breeding pairs of PAR2–/– mice. We thank R. Ruthazer, Biostatistics Research Center at Tufts Medical Center, for help with the statistical analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants AA015410 (to G. P.) and DK031396 (to M. L. S.), This work was also supported by a fellowship from Sigrid Jusélius Foundation, Finland (J. Laukkarinen), the Stichting Prof. Michaël-van Vloten Fonds in the Netherlands, and the Netherlands Organisation for Health Research and Development ZonMw, MD-Medical Research Trainee Grant 920-03-327 (to G. J. D. v. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
Footnotes
The abbreviations used are: PAR2, protease-activated receptor-2; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; SBTI, soybean trypsin inhibitor; TLCS, taurolithocholic acid 3-sulfate disodium salt; MAP, mitogen-activated protein.
References
- 1.Lampel, M., and Kern, H. F. (1977) Virchows Arch. A. Pathol. Anat. Histol. 373 97–117 [DOI] [PubMed] [Google Scholar]
- 2.Aho, H. J., Koskensalo, S. M., and Nevalainen, T. J. (1980) Scand. J. Gastroenterol. 15 411–416 [DOI] [PubMed] [Google Scholar]
- 3.Laukkarinen, J. M., van Acker, G. J., Weiss, E., Steer, M. L., and Perides, G. (2007) Gut 56 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Acker, G. J., Perides, G., Weiss, E. R., Das, S., Tsichlis, P. N., and Steer, M. L. (2007) J. Biol. Chem. 282 22140–22149 [DOI] [PubMed] [Google Scholar]
- 5.Wittel, U. A., Wiech, T., Chakraborty, S., Boss, B., Lauch, R., Batra, S. K., and Hopt, U. T. (2008) Pancreas 36 e9–21 [DOI] [PubMed] [Google Scholar]
- 6.Namkung, W., Han, W., Luo, X., Muallem, S., Cho, K. H., Kim, K. H., and Lee, M. G. (2004) Gastroenterology 126 1844–1859 [DOI] [PubMed] [Google Scholar]
- 7.Sharma, A., Tao, X., Gopal, A., Ligon, B., Andrade-Gordon, P., Steer, M. L., and Perides, G. (2005) Am. J. Physiol. 288 G388–G395 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen, T. D., Moody, M. W., Steinhoff, M., Okolo, C., Koh, D. S., and Bunnett, N. W. (1999) J. Clin. Investig. 103 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ossovskaya, V. S., and Bunnett, N. W. (2004) Physiol. Rev. 84 579–621 [DOI] [PubMed] [Google Scholar]
- 10.Singh, V. P., Bhagat, L., Navina, S., Sharif, R., Dawra, R. K., and Saluja, A. K. (2007) Gut 56 958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano, B. P., Cheung, W. M., Santulli, R. J., Fung-Leung, W. P., Ngo, K., Ye, R. D., Darrow, A. L., Derian, C. K., de Garavilla, L., and Andrade-Gordon, P. (1999) J. Pharmacol. Exp. Ther. 288 671–678 [PubMed] [Google Scholar]
- 12.Frossard, J. L., Saluja, A., Bhagat, L., Lee, H. S., Bhatia, M., Hofbauer, B., and Steer, M. L. (1999) Gastroenterology 116 694–701 [DOI] [PubMed] [Google Scholar]
- 13.Sharma, A., Tao, X., Gopal, A., Ligon, B., Steer, M. L., and Perides, G. (2005) Am. J. Physiol. 289 G686–G695 [DOI] [PubMed] [Google Scholar]
- 14.Leach, S. D., Modlin, I. M., Scheele, G. A., and Gorelick, F. S. (1991) J. Clin. Investig. 87 362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, L., Gukovskaya, A. S., Penninger, J. M., Mareninova, O. A., Friess, H., Gukovsky, I., and Pandol, S. J. (2007) Am. J. Physiol. 292 G875–G886 [DOI] [PubMed] [Google Scholar]
- 16.Sjodin, L., Dahlen, H. G., and Gylfe, E. (1991) J. Physiol. (Lond.) 444 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yule, D. I., and Williams, J. A. (1992) J. Biol. Chem. 267 13830–13835 [PubMed] [Google Scholar]
- 18.Lignon, M. F., Galas, M. C., Rodriguez, M., Gullion, G., Jard, S., and Martinez, J. (1988) in Gastrin and Cholecystokinin, Chemistry, Physiology and Pharmacology (Bali, J. P., and Martinez, J., eds) pp. 57–60, Elsevier Science Publishing Co., Inc., New York
- 19.Kim, J. Y., Kim, K. H., Lee, J. A., Namkung, W., Sun, A. Q., Ananthanarayanan, M., Suchy, F. J., Shin, D. M., Muallem, S., and Lee, M. G. (2002) Gastroenterology 122 1941–1953 [DOI] [PubMed] [Google Scholar]
- 20.Mooren, F., Hlouschek, V., Finkes, T., Turi, S., Weber, I. A., Singh, J., Domschke, W., Schnekenburger, J., Kruger, B., and Lerch, M. M. (2003) J. Biol. Chem. 278 9361–9369 [DOI] [PubMed] [Google Scholar]
- 21.Saluja, A. K., Donovan, E. A., Yamanaka, K., Yamaguchi, Y., Hofbauer, B., and Steer, M. L. (1997) Gastroenterology 113 304–310 [DOI] [PubMed] [Google Scholar]
- 22.Grady, T., Mah'Moud, M., Otani, T., Rhee, S., Lerch, M. M., and Gorelick, F. S. (1998) Am. J. Physiol. 275 G1010–G1017 [DOI] [PubMed] [Google Scholar]
- 23.Steer, M. L., and Perides, G. (2005) in Clinical Pancreatology for Practicing Gastroenterologists and Surgeons (Munoz, E. D., and Paraskeva, K., eds) pp. 10–26, Blackwell Publishing, Oxford, UK
- 24.Acosta, J. M., and Ledesma, C. L. (1974) N. Engl. J. Med. 290 484–487 [DOI] [PubMed] [Google Scholar]
- 25.Glasbrenner, B., and Adler, G. (1993) Hepatogastroenterology 40 517–521 [PubMed] [Google Scholar]
- 26.Opie, E. L. (1901) Bull. Johns Hopkins Hosp. 12 182–192 [Google Scholar]
- 27.Opie, E. L. (1901) Am. J. Med. Surg. 12 27–40 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.