Abstract
RecQ helicases are found in organisms as diverse as bacteria, fungi, and mammals. These proteins promote genome stability, and mutations affecting human RecQ proteins underlie premature aging and cancer predisposition syndromes, including Bloom syndrome, caused by mutations affecting the BLM protein. In this study we show that mutants lacking the Rqh1 protein of the fission yeast Schizosaccharomyces pombe, a RecQ and BLM homolog, have substantially reduced meiotic recombination, both gene conversions and crossovers. The relative proportion of gene conversions having associated crossovers is unchanged from that in wild type. In rqh1 mutants, meiotic DNA double-strand breaks are formed and disappear with wild-type frequency and kinetics, and spore viability is only moderately reduced. Genetic analyses and the wild-type frequency of both intersister and interhomolog joint molecules argue against these phenotypes being explained by an increase in intersister recombination at the expense of interhomolog recombination. We suggest that Rqh1 extends hybrid DNA and biases the recombination outcome toward crossing over. Our results contrast dramatically with those from the budding yeast ortholog, Sgs1, which has a meiotic antirecombination function that suppresses recombination events involving more than two DNA duplexes. These observations underscore the multiple recombination functions of RecQ homologs and emphasize that even conserved proteins can be adapted to play different roles in different organisms.
HOMOLOGOUS recombination allows the faithful repair of DNA double-strand breaks (DSBs) by generating a joint molecule between the broken DNA and an intact homologous duplex. In this joint molecule, the intact duplex provides a template for repair of the broken DNA. This repair can lead to gene conversion events, which may or may not be accompanied by a crossover between the two interacting duplexes. Although many of the DNA intermediates and protein effectors of homologous recombination are now known, the regulation of this process is still poorly understood.
Although homologous recombination provides a method to faithfully repair DSBs, there are dangers associated with this process. Genome rearrangements or loss of heterozygosity can occur during mitotic growth if homologous recombination generates crossovers. This danger is lessened if recombination is directed to preferentially avoid crossovers. However, the danger can also be avoided by the use of alternative DNA repair mechanisms, instead of homologous recombination. In contrast to mitotic growth, during meiotic recombination DSBs are actively generated by the cell, using the Rec12 (Spo11) protein, precisely to produce crossovers. Therefore, depending on the situation, it may be advantageous for cells to promote or discourage the use of homologous recombination over other DNA repair mechanisms or to promote certain outcomes of homologous recombination over others (e.g., crossovers vs. noncrossovers).
Recent work has suggested that RecQ helicases are critical to understanding the interplay of pro- and antirecombination factors in DSB repair. RecQ helicases are a conserved family of proteins that unwind DNA with a 3′ → 5′ polarity and are found in organisms as diverse as mammals, fungi, and bacteria (reviewed by Cobb and Bjergbaek 2006). Mutations affecting RecQ proteins often lead to genome instability and associated diseases, such as cancer. In humans, there are five RecQ helicases, mutations affecting three of which, the BLM, WRN, and RECQL4 proteins, are associated with genetic instability and cancer predisposition (e.g., Puranam and Blackshear 1994; Ellis et al. 1995; Yu et al. 1996; Kitao et al. 1999; reviewed by Hanada and Hickson 2007).
There are several lines of evidence indicating that members of the RecQ family have an antirecombination function, particularly in the suppression of “aberrant” recombination events. For instance, patients with Bloom syndrome (caused by mutations in BLM) have a greatly elevated frequency of chromosome rearrangements and breaks, which in turn can explain their predisposition to developing cancer (e.g., Chaganti et al. 1974; Ellis et al. 1995; reviewed by Hanada and Hickson 2007). Sgs1 and Rqh1 are BLM homologs found in budding and fission yeast, respectively. All of these proteins have a domain structure consisting of highly acidic regions, a helicase domain, a helicase C-terminal domain, and an helicase RNase D C terminus (HRDC) domain. Mutations affecting these proteins are also associated with elevated levels of homologous recombination during mitotic growth (Watt et al. 1996; Stewart et al. 1997). In addition, double mutants with sgs1 (or rqh1) and srs2, encoding another 3′ → 5′ helicase, have very low viability. This last phenotype is suppressed by mutations altering the Rad51 strand-exchange protein, suggesting that the inviability of the double sgs1 srs2 and rqh1 srs2 mutants is caused by the accumulation of toxic recombination intermediates (Gangloff et al. 2000; Maftahi et al. 2002; Liberi et al. 2005).
The Srs2 helicase is structurally related to the UvrD helicase of bacteria, rather than to the RecQ family. This protein is found in both budding and fission yeast, but no mammalian homologs have been identified (Lawrence and Christensen 1979; Wang et al. 2001). The function of Srs2 often seems to be closely linked to that of Sgs1 or Rqh1. Both Sgs1 (Rqh1) and Srs2 proteins have an antirecombination function during mitotic growth of budding and fission yeast (Rong et al. 1991; Watt et al. 1996; Stewart et al. 1997; Wang et al. 2001; Doe and Whitby 2004; J. Virgin, personal communication). In addition, both Sgs1 (Rqh1) and budding yeast Srs2 promote noncrossover outcomes over crossover outcomes during mitotic recombination, implicating them in this aspect of recombination regulation (Ira et al. 2003; Hope et al. 2007; J. Virgin, personal communication). The elevated-crossover phenotype of either mutant can be suppressed by overexpression of the other protein, and the sensitivity to DNA damaging agents of srs2 mutants can be suppressed by overexpression of SGS1 (Mankouri et al. 2002; Ira et al. 2003).
The antirecombination function of RecQ proteins (and Srs2) is not fully understood. However, one possibility is that their helicase action reverses or destabilizes recombination intermediates, such as Holliday junctions (HJs). This reversal would prevent HJs being resolved to give crossovers and hence would reduce crossover recombination frequencies and the proportion of crossover to noncrossover outcomes of recombination. Consistent with this model, mutations affecting both Sgs1 (Rqh1) and Mus81, a component of the Mus81-Eme1 HJ-resolving enzyme, are synthetically lethal (Boddy et al. 2000; Mullen et al. 2001), and overexpression of a bacterial HJ resolvase can partially suppress some rqh1 mutant phenotypes, such as sensitivity to DNA damaging agents (Doe et al. 2000). In further support of this view, the helicase activity of the BLM protein, in conjunction with topoisomerase IIIα and the Bloom syndrome complex protein BLAP75, can disassemble double HJs in vitro (Wu et al. 2006). The Sgs1 protein, without accessory factors, can also unwind simple HJ structures in vitro (Bennett et al. 1999). However, mutations affecting budding yeast Top3, the topoisomerase IIIα ortholog, have phenotypes similar to those of sgs1 mutants, supporting a DNA processing pathway involving both proteins rather than Sgs1 alone (Ira et al. 2003; Liberi et al. 2005). In fission yeast, Rqh1 also interacts with the Top3 ortholog (Ahmad and Stewart 2005).
At least in budding yeast, RecQ proteins also have an antirecombination function during meiosis. Budding yeast sgs1 mutants show mildly elevated frequencies of meiotic crossovers, apparently due to the formation of recombination intermediates involving more than two homologous duplexes, a unique phenotype (Rockmill et al. 2003; Jessop et al. 2006; Oh et al. 2007). This antirecombination activity of Sgs1 appears to be antagonized by a prorecombination activity of the ZMM group of proteins (Jessop et al. 2006; Oh et al. 2007), which appears to be specifically required for the formation of one subset of meiotic crossovers in budding yeast (reviewed by Cromie and Smith 2007; Lynn et al. 2007). The recombination deficiency of several zmm mutants is at least partially suppressed by a further mutation in SGS1 (Jessop et al. 2006; Oh et al. 2007). Mutation of budding yeast SRS2 reduces meiotic spore viability, but this phenotype is not suppressed by additional mutations in SPO13 and MEI4, which bypass meiosis I and DSB formation and repair (Palladino and Klein 1992). This result suggests that budding yeast srs2 mutants have a meiotic defect unrelated to meiotic recombination.
We set out to investigate the roles of Rqh1 and Srs2 in fission yeast meiosis. Despite the similar mitotic roles of fission yeast Rqh1 and budding yeast Sgs1, it seemed plausible that the meiotic roles of these proteins would be distinct, because fission yeast lacks the ZMM proteins. In fact, we observe that rqh1 mutants are recombination deficient, in complete contrast to the situation in budding yeast, where sgs1 mutants have elevated recombination frequencies. We see little effect of the srs2 mutation on recombination. The recombination deficiency of the rqh1 mutant is not explained by a failure to generate or repair DSBs, and spore viability in this genetic background is only moderately reduced. Genetic and physical assays argue against the simplest explanation for these phenotypes: that DSBs are repaired more frequently against sister chromatids, rather than homologous chromosomes, in an rqh1 mutant. We suggest that, for meiotic DSB repair, Rqh1 is required to extend hybrid DNA and to bias the outcome toward crossing over.
MATERIALS AND METHODS
Yeast strains and genetic techniques:
The Schizosaccharomyces pombe strains used in this study, and their genotypes, are listed in Table 1. Meiotic crosses were carried out by suspending single yeast colonies in 5 ml of supplemented yeast extract liquid medium [YEL + adenine (100 μg/ml)] (Smith 2008) and growing at 30° until saturated. For each cross, aliquots of 100 μl from two saturated cultures were mixed, and the cells were washed twice with water and spotted on sporulation agar plates (SPA) (Smith 2008) supplemented with any required amino acids, purines, and pyrimidines. After 2 days of incubation at 25° the cell–ascus mixture from each spot was suspended in 1 ml of water and treated with glusulase and ethanol to kill vegetative cells, essentially as described by DeVeaux et al. (1992).
TABLE 1.
S. pombe strains used in this study
| Strain | Genotypea |
|---|---|
| GP2 | h− |
| GP6 | h+ ade6-M375 |
| GP13 | h− ade6-52 |
| GP14 | h+ ade6-52 |
| GP19 | h+ |
| GP23 | h− ade6-M26 |
| GP24 | h+ ade6-M26 |
| GP64 | h− pat1-114 |
| GP65 | h+ pat1-114 |
| GP747 | h− ura1-171 |
| GP850 | h+ lys3-37 ura1-61 |
| GP1040 | h− ura4-D18 ade6-52 ura4A+ |
| GP4297 | h+ ade6-3049 |
| GP4298 | h− ade6-3049 |
| GP5086 | h−/h− ura1-61/+ mbs1-25/mbs1-24 pat1-114/pat1-114 ade6-210/ade6-216 |
| GP5263 | h+ pat1-114 rqh1∷kanR |
| GP5348 | h+ lys3-37 ura1-61 srs2∷kanR |
| GP5350 | h+ lys3-37 ura1-61 rqh1∷kanR |
| GP5352 | h− srs2∷kanR |
| GP5353 | h+ srs2∷kanR |
| GP5354 | h− ura1-171 srs2∷kanR |
| GP5355 | h+ rqh1∷kanR |
| GP5356 | h− rqh1∷kanR |
| GP5357 | h− ura1-171 rqh1∷kanR |
| GP5489 | h− pat1-114 rqh1∷kanR |
| GP5493 | h− ura4-D18 ade6-52 ura4A+ rqh1∷kanR |
| GP5494 | h− ura4-D18 ade6-52 ura4A+ srs2∷kanR |
| GP5495 | h+ ura4-D18 ade6-M26 tps16-23barg1-14 |
| GP5557 | h+ ura4-D18 ade6-M26 tps16-23barg1-14 rqh1∷kanR |
| GP5559 | h+ ura4-D18 ade6-M26 tps16-23barg1-14 srs2∷kanR |
| GP5835 | h− ade6-Dup(M26-ura4+-469) ura4-D18 rqh1∷kanR |
| GP5837 | h− ade6-Dup(M26-ura4+-469) ura4-D18 |
| GP5859 | h+ ade6-D19 leu1-32 his3-D1 ura4-D18 rqh1∷kanR |
| GP5860 | h+ ade6-D19 leu1-32 his3-D1 ura4-D18 |
| GP5868 | h+ leu1-32 rec12-152∷LEU2 |
| GP5869 | h− leu1-32 rec12-152∷LEU2 |
| GP6318 | h− ade6-M375 |
| GP6489 | h+/h+ ura1-61/+ +/lys3-37 mbs1-25/mbs1-24 pat1-114/pat1-114 ade6-210/ade6-216 rqh1∷kanR/rqh1∷kanR |
| GP6608 | h+ ade6-M26 rqh1∷kanR |
| GP6609 | h− ade6-M26 rqh1∷kanR |
| GP6610 | h+ ade6-M375 rqh1∷kanR |
| GP6611 | h− ade6-M375 rqh1∷kanR |
| GP6612 | h+ ade6-52 rqh1∷kanR |
| GP6613 | h− ade6-52 rqh1∷kanR |
| GP6614 | h+ ade6-3049 rqh1∷kanR |
| GP6615 | h− ade6-3049 rqh1∷kanR |
Mutations other than commonly used auxotrophies and mating-type alleles are described in the following references: ade6-Dup(M26-ura4+-469) (Schuchert and Kohli 1988), mbs1 alleles (Cromie et al. 2006), pat1-114 (Iino and Yamamoto 1985), rqh1∷kanR and srs2∷kanR (Maftahi et al. 2002), tps16-23 (Gygax and Thuriaux 1984), ura4A+ (Zahn-Zabal et al. 1995), and rec12-152∷LEU2 (Lin and Smith 1994).
tps16 mutations map to the ags1 gene (F. Hochstenbach, personal communication).
For measurement of viable spore yield, crosses were carried out as above, and the titers of the two cultures used for the meiotic cross were measured at the time of crossing by assay on appropriately supplemented YEA plates. The titer of viable spores was measured in the final spore suspension. The viable spore yield was calculated as the number of viable spores per viable cell in the mated culture with the lower viable cell titer (Ellermeier et al. 2004).
For measurement of spore viability, spore suspensions were prepared essentially as described above, but with spore release by autolysis (i.e., without glusulase or ethanol treatment). The cell–ascus–spore suspension was spotted onto YEA-5S (YEA + adenine, uracil, lysine, histidine, and leucine) plates, and individual spores were micromanipulated under a microscope onto a grid on another part of the plate. Plates were incubated at 32° for 4 days, and the proportion of spores that had formed visible colonies was calculated.
Auxotrophic markers were scored by transferring well-isolated yeast colonies to appropriately supplemented YEA plates, followed by replica-plating onto appropriately supplemented nitrogen-base minimal agar (NBA) (Smith 2008). Replica plating onto YEA + phloxin B plates (Moreno et al. 1991) at 37° was used to score tps16.
Measurement of gene conversion at ade6 and associated crossovers:
Spores with gene conversion at the ade6 or ura1 loci were selected as intragenic recombinants (prototrophs) in crosses between different ade6 and ura1 point mutants (see Smith 2008 and results section for rationale). Measurement of total viable spores and prototrophic spores, per unit volume of spore suspension, allowed calculation of gene conversion frequencies. Crossovers of flanking markers (ura4A+ and tps16) accompanying gene conversion at ade6 were measured among gene convertant spores, selected as above.
Determination of sister-chromatid recombination frequencies:
Combined intrachromatid and unequal intersister–chromatid exchange frequencies were determined as follows. Appropriately diluted mitotic cultures of the ade6-Dup-containing strain were plated on YEA + adenine to determine the total number of viable cells and on YEA + guanine to determine the frequency of mitotic Ade+ recombinants (Schuchert and Kohli 1988; Davis and Smith 2006). The ade6-Dup strain and the appropriate ade6-D19 (complete ade6 deletion) strain were then mated on supplemented SPA. Spores were harvested, and spore suspensions were plated on YEA + adenine to determine the total frequency of viable cells and on YEA + guanine to determine the frequency of Ade+ recombinants. The mitotic frequency was subtracted from the meiotic frequency to give the final meiotic intra- and intersister recombinant frequency. Four crosses were performed for each genotype, and the statistical significance was calculated using Student's t-test.
Analysis of meiotic DNA breaks:
Meiotic inductions, flow cytometry, and preparation of DNA in agarose plugs were performed as described by Young et al. (2002). The agarose-embedded DNA was digested with restriction enzymes, separated by gel electrophoresis, Southern blotted, and hybridized with probe c139 (Young et al. 2002).
Detection and quantitation of crossover molecules and intersister and interhomolog joint molecules:
Digestion of DNA in agarose plugs, separation by electrophoresis on one- or two-dimensional gels, Southern blotting, probing, and quantitation were carried out as described by Cromie et al. (2006) and Hyppa and Smith (2008).
RESULTS
rec9-104 is an allele of rqh1, with a mutation in the helicase C-terminal domain:
The rec9-104 mutation reduces the frequencies of meiotic gene conversion and crossing over approximately fivefold (Ponticelli and Smith 1989; DeVeaux et al. 1992). Gene conversion is measured as ade6 intragenic recombination and crossing over as intergenic recombination (for justification see below and Gutz 1971; P. Munz, personal communication cited in Young et al. 2002; Smith 2008). The rec9-104 allele is linked to ura1 and fails to complement the meiotic recombination defect of rqh1 (also known as hus2) mutants, identifying rec9-104 as an allele of rqh1 (Davis and Smith 2001; J. Young and G. Smith, unpublished data). The rec9-104 allele contains a missense mutation (G → A at position 2264 in the coding sequence) leading to the change Cys → Tyr at residue 755 of the protein, in the helicase C-terminal domain (C. Ellermeier and G. Smith, unpublished data).
Gene conversion and crossover frequencies are substantially reduced in an rqh1 mutant but not in an srs2 mutant:
Meiotic recombination phenotypes of rqh1 mutants have been characterized only for the rec9-104 allele, which may affect only one of the domains of the multidomain Rqh1 protein. Therefore, we began our investigation of the meiotic roles of Rqh1 by examining the effect of an rqh1 null mutation (a complete deletion of the rqh1 coding sequence) on meiotic recombination frequency. In addition, we examined the effect of an srs2 null mutation because of the known interaction between Rqh1 (Sgs1) and Srs2 in mitotic growth and the role of both Rqh1 (Sgs1) and Srs2 proteins in promoting mitotic noncrossover vs. crossover events (Gangloff et al. 2000; Maftahi et al. 2002; Ira et al. 2003; Doe and Whitby 2004; Liberi et al. 2005; Hope et al. 2007; J. Virgin, personal communication).
Meiotic crossover frequencies were measured for three intervals, the ura4A+–tps16 and tps16–arg1 intervals of chromosome III (ura4A+ is also known as ura4+-aim) and the lys3–ura1 interval of chromosome I. In each case, crossover frequencies (cM) were reduced to 10–20% of wt (rqh1+) levels in the rqh1 mutant and to 60–80% of wt levels in the srs2 mutant (Figure 1A). The differences between wt and rqh1Δ recombination frequencies were significant (P < 0.0001 in all cases; Fisher's exact test), but those between wt and srs2Δ were significant only for the ura4A+–tps16 interval (P < 0.01; Fisher's exact test).
Figure 1.—
srs2 and rqh1 null mutants have reduced crossover and gene conversion frequencies. (A) Crossover frequencies were measured in three intergenic intervals among the products of meiotic crosses and converted to centimorgans by the method of Haldane (1919). Data represent combined numbers from at least two independent experiments (537–600 spore colonies, total, for each cross), normalized to the wt genetic distance. Error bars indicate 95% binomial proportion confidence intervals calculated by the Wilson score interval method (Wilson 1927). The strains used for the lys3–ura1 measurements were: GP2 and GP850 (wt), GP5348 and GP5352 (srs2Δ), GP5350 and GP5356 (rqh1Δ). The strains used for both the ura4A+–tps16 and the tps16–arg1 measurements were: GP1040 and GP5495 (wt), GP5494 and GP5559 (srs2Δ), GP5493 and GP5557 (rqh1Δ). The wt genetic distances for lys3–ura1, ura4A+–tps16, and tps16–arg1 were 25, 17, and 55 cM, respectively. (B) Gene conversion frequencies at ade6 and ura1 were measured as the frequency of prototrophs in meiotic crosses involving point mutations of each gene (ade6-52 × ade6-M26 and ura1-61 × ura1-171). Data are the average of three independent experiments, normalized to the prototroph frequency observed in the wt crosses. Error bars indicate SEM (n = 3). The strains used in crosses for the ade6 measurements were: GP1040 and GP5495 (wt), GP5494 and GP5559 (srs2Δ), GP5493 and GP5557 (rqh1Δ). The strains used in crosses for the ura1 measurements were: GP850 and GP747 (wt), GP5348 and GP5354 (srs2Δ), GP5350 and GP5357 (rqh1Δ). The wt prototroph frequencies, per viable spore, for ura1 and ade6 were 0.00020 and 0.0033, respectively.
Gene conversion frequencies were measured as intragenic recombination frequencies at two loci: ura1 on chromosome I and ade6 on chromosome III. For intragenic markers spanning most of ade6, all of 123 recombinants tested arose from gene conversion; none arose from crossing over (Gutz 1971). In the rqh1 mutant, gene conversion frequencies were significantly reduced to 10–20% of wt levels (P < 0.01 in both cases; Student's t-test, two tailed), similar to the reduction in crossover frequencies. In the srs2 mutant, effects on ura1 and ade6 gene conversion frequencies were modest and of marginal significance (P = 0.028 and P = 0.085, respectively; Student's t-test, two tailed), and no consistent direction of effect was observed (Figure 1B). To test for any contribution of marker effects on the rqh1 mutant phenotype, intragenic recombination frequencies were measured at ade6 for three pairs of markers. A similar reduction in recombination frequencies, by a factor of 6–10, was seen for all three marker combinations in the rqh1 mutant compared to wt (Table 2).
TABLE 2.
rqh1 null mutation reduces intragenic recombination at ade6 similarly for three different allele-pair combinations
| Recombination frequency (Ade+ per 106 viable spores)
|
Mean fold-reduction in rqh1Δ | ||
|---|---|---|---|
| ade6 markers | wt | rqh1Δ | |
| M375 × 52 | 180, 240 | 18, 25 | 10 |
| M26 × 52 | 1,800, 1,800 | 390, 250 | 6 |
| M26 × 3049 | 17,000, 16,000 | 3,000, 2,200 | 6 |
For each genotype and combination of markers two crosses were done, and the ade6+ recombinant frequency measured. Crosses were: (top row, left to right) GP6318 × GP14, GP6 × GP13, GP6611 × GP6612, GP6610 × GP6613; (middle row, left to right) GP23 × GP14, GP24 × GP13, GP6609 × GP6612, GP6608 × GP6613; (bottom row, left to right) GP23 × GP4297, GP24 × GP4298, GP6609 × GP6614, GP6608 × GP6615. Additional ade6-M26 × ade6-52 data are shown in Figure 1B.
The rqh1 mutant values are similar to those observed in both inter- and intragenic crosses with rec9-104 (Ponticelli and Smith 1989; DeVeaux et al. 1992), suggesting that rec9-104 is a null mutation with respect to its effect on meiotic crossing over and gene conversion.
Possible explanations for the recombination deficiency of rqh1 mutants:
Crossovers occur when meiotic DSBs are repaired against homologous chromosomes, with appropriate resolution of the Holliday junction(s) in the DNA intermediates. The reduction in crossover frequencies seen in the rqh1 mutant could therefore have several explanations. DSB frequency could be reduced, or a proportion of DSBs could remain unrepaired. Repair could occur between identical sister chromatids, rather than homologous chromosomes, a process that cannot produce genetic crossovers. Alternatively, repair could still occur against the homologous chromosome but by mechanisms such as synthesis-dependent strand annealing (SDSA) that avoid Holliday junctions and produce only noncrossovers. We set out to test these possibilities.
The relative frequencies of crossover and noncrossover recombination are not altered in rqh1 or srs2 mutants:
One of the mitotic phenotypes of both rqh1 and srs2 mutants is an increase in crossover vs. noncrossover outcomes to recombination (Ira et al. 2003; Hope et al. 2007; J. Virgin, personal communication). As discussed above, if the rqh1 mutant had the opposite effect in meiosis, this would explain the reduced levels of crossovers seen in this background. Therefore, we examined the effect of the rqh1 and srs2 null mutants on the relative frequencies of these two outcomes during meiotic recombination.
When a DSB is repaired by recombination, gene conversion can occur with or without an associated crossover. The proportion of gene conversions at a particular site that have an associated crossover therefore gives an estimate of the proportion of all recombination events at that site that give rise to crossovers (but see discussion). In wt meiotic crosses, when a gene conversion occurs at ade6, ∼65% of the time it is accompanied by a crossover between the flanking markers ura4A+ and tps16 (Grimm et al. 1994; Cromie et al. 2005). There is no significant change in this frequency in either the srs2 or rqh1 mutant backgrounds (P = 0.34 and P = 0.62, respectively; Fisher's exact test; Figure 2). Therefore, neither Rqh1 nor Srs2 appears to regulate the frequency of crossover vs. noncrossover recombination during meiosis. This conclusion is further supported by the observation that, in both the rqh1 and srs2 mutant backgrounds, gene conversion frequencies are affected to a similar extent as crossover frequencies.
Figure 2.—
The srs2 and rqh1 null mutations have no effect on the frequency with which gene conversions at ade6 are accompanied by crossovers between flanking markers. Gene convertants were selected as prototrophs arising in meiotic crosses involving the ade6-52 and ade6-M26 point mutations. Among these gene convertants (589–605 spore colonies, total, for each cross), the percentage of spores displaying crossovers between the markers ura4A+ and tps16, flanking ade6, was measured. Percentages represent combined data from two independent experiments. Error bars indicate 95% binomial proportion confidence intervals calculated by the Wilson score interval method. The strains used for the crosses were: GP1040 and GP5495 (wt), GP5494 and GP5559 (srs2Δ), GP5493 and GP5557 (rqh1Δ).
Spore viability is only moderately reduced in an rqh1 mutant:
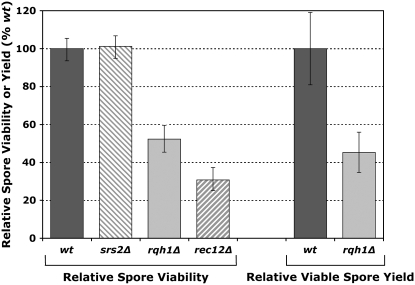
Defects in meiotic DSB formation or repair are expected to result in reduced spore viability, with DSB-repair mutants showing the greater impairment (reviewed in Cromie and Smith 2008). To help identify the nature of their respective recombination defects, we tested the effect of the rqh1 and srs2 null mutations on spore viability. The srs2 mutation had essentially no effect (P = 0.84; Fisher's exact test), consistent with its mild effect on meiotic recombination. The rqh1 mutation had a more pronounced, and significant, defect, with spore viability reduced to ∼50% of wt levels (P < 0.0001; Fisher's exact test). However, this effect is considerably weaker than that in mutants, such as rec12 (P < 0.0001; Fisher's exact test), that fail to form breaks (Figure 3; Davis and Smith 2003) and may be exaggerated by the mild mitotic growth defect of rqh1Δ cells (Stewart et al. 1997; our unpublished data). The moderate spore viability defect of the rqh1 mutant is consistent with a chromosome segregation problem, expected due to the reduced levels of crossing over seen in the mutant (Figure 1A).
Figure 3.—
The rqh1 null mutation has moderately reduced spore viability and viable spore yield, while there is no significant effect of the srs2 null mutation on spore viability. Left: spore viability was measured as the percentage of spores, relative to wt, successfully germinating into a visible colony after gridding by micromanipulation on YEA-5S plates. The wt spore viability was 78%. From each of two independent crosses 152 spores were micromanipulated for each genotype. Error bars indicate 95% binomial proportion confidence intervals calculated by the Wilson score interval method using the combined data. Crosses were GP2 × GP19 (wt), GP5352 × GP5353 (srs2Δ), GP5355 × GP5356 (rqh1Δ), and GP5868 × GP5869 (rec12Δ). Right: viable spore yield was measured as the number of viable spores produced per number of cells of the less numerous parent in the cross. The strains used were GP2 × GP19 (wt) and GP5355 × GP5356 (rqh1Δ). Data, expressed as a percentage of rqh1+, which produced 7.2 spores per less numerous cell, are the mean of at least four independent experiments ± SEM.
Viable spore yield, measured as the number of viable spores produced per cell in the mating mixture, was also reduced to ∼50% of wt levels in the rqh1 mutant, similar to the effect on spore viability (Figure 3). This similarity suggests that the rqh1 mutant does not have a substantial defect in mating or sporulation.
Meiotic DSBs are formed at normal frequencies and repaired with normal kinetics in the rqh1 null mutant:
Mutants that fail to repair meiotic DSBs, such as those affecting the MRN complex, have a lower spore viability than those, such as rec12 mutants, that do not form breaks at all (Tavassoli et al. 1995; Davis and Smith 2003; Young et al. 2004). As the rqh1 mutant had a higher spore viability than that of the rec12 mutant, this suggests that the reduced spore viability seen in the rqh1 mutant is not caused by a failure to make or repair meiotic DSBs. Rather, it is consistent with the slight chromosome-segregation defect expected due to reduced crossover frequencies in the rqh1 mutant background.
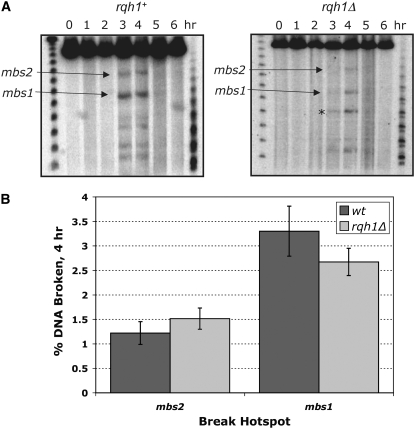
This idea was tested directly by examining the formation and repair of meiotic DSBs in wt and rqh1 mutant cultures induced to undergo meiosis. Meiotic DSBs appeared and disappeared at the same times and at approximately the same frequencies in both genetic backgrounds (Figure 4). The cumulative frequency of meiotic DSBs in the rqh1 mutant could not be measured because the rad50S allele, often used to accumulate meiotic DSBs (e.g., Young et al. 2002), leads to poor viability and slow growth of cultures when combined with the rqh1 null mutation (our unpublished data). Nevertheless, the rad50+ results indicate that DSBs are formed and repaired in the rqh1 null mutant at a similar frequency as in wt. As expected from the mild phenotypes reported above, breaks are also formed and repaired normally in the srs2 mutant (our unpublished data).
Figure 4.—
DSB formation and repair are normal in the rqh1 null mutant. (A) Southern blots of NotI fragment J on chromosome I, probed from the right side, showing formation and repair of DSBs at the mbs1 and mbs2 meiotic DSB hotspots in the wt and rqh1Δ backgrounds. Synchronous meiosis was induced by temperature-shifting pat1-114 cultures, and DNA, isolated at the times indicated, was analyzed as described by Young et al. (2002). The strains used were GP65 (wt) and GP5263 (rqh1Δ). The asterisk indicates an additional break site introduced by the kanR substitution at the rqh1 locus ∼70 kb from mbs1. The markers are MidRange I PFG (right) and Lambda Ladder PFG marker (left) (New England Biolabs). (B) DSB frequencies at the mbs1 and mbs2 hotspot sites, from DNA samples harvested 4 hr after meiotic induction (when breaks are close to maximal) and probed as in Figure 4A. Quantitation was done using a phosphorimager. For each genetic background, values are from one h+ and one h− induction, each assayed on multiple Southern blots. The mean ± SEM is shown; n = 5 for wt and 7 for rqh1Δ. The strains used were: GP65 and GP64 (wt); GP5263 and GP5489 (rqh1Δ).
Intersister recombination is not elevated in an rqh1 mutant:
The simplest explanation for the phenotypes of the rqh1 null mutant described above is that, in the mutant, there is a specific defect in repairing DSBs using the homologous chromosome, and instead breaks are repaired using other homologous targets, such as the sister chromatid. Intersister recombination would explain the high spore viability and repair of meiotic DSBs without leading to detectable crossovers and gene conversions. This is consistent with mitotic studies, where rqh1 mutants show elevated levels of intersister recombination (Hope et al. 2006).
Two assays were used to test this hypothesis. First, we used a genetic assay based on recombination between duplicated DNA sequences to measure the frequency of intersister (or intrachromosomal) recombination, i.e., noninterhomolog recombination, in wt and rqh1 mutant backgrounds (Figure 5). In wt this assay produces about equal numbers of recombinants through unequal exchange (intersister) and “popout” (intrasister) events (Schuchert and Kohli 1988). No elevation of Ade+ recombinant frequency was seen by this assay in the rqh1 mutant compared to wt. The observed reduction was not statistically significant (P = 0.36; Student's t-test; Figure 5).
Figure 5.—
Frequency of meiotic recombination between tandemly duplicated copies of the ade6 gene, one marked with M26 and the other with 469, to give Ade+ spores. In this assay, recombination cannot use the homologous chromosome as a template, since ade6 is deleted from one parent. The tandem 1.9-kb duplications of ade6 are separated by ∼4.4 kb of vector DNA and the ura4 gene, cloned into the pUC18 vector (Schuchert and Kohli 1988). The strains used were GP5837 and GP5860 (wt) and GP5835 and GP5859 (rqh1Δ). Data are the mean of four independent experiments ± SEM.
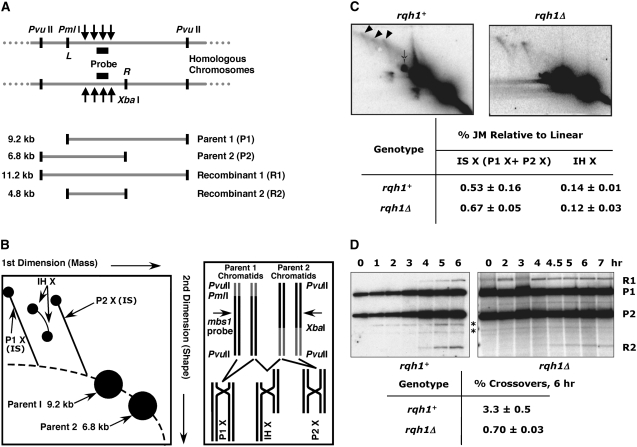
A second, physical assay was used to examine directly the effect of the rqh1 null mutation on the relative frequency of recombination intermediates involving sister chromatids vs. homologous chromosomes. Diploids with heterozygous restriction sites flanking the mbs1 DSB and recombination hotspot (Cromie et al. 2005, 2006) allow intersister and interhomolog recombination intermediates at this site to be detected and distinguished from one another. This is done by isolation of DNA from meiotically induced diploids after DSB formation, followed by restriction enzyme digestion, resolution on 2-D gels, and probing for mbs1 (Cromie et al. 2006; Hyppa and Smith 2008). The 2-D gels separate DNA based mostly on molecular mass in the first dimension and on both molecular mass and shape in the second dimension. Parental DNA and intersister and interhomolog joint molecules run at distinctive positions on these gels (Figure 6, A and B). In wt, recombination-derived joint molecules are maximal at ∼4.5 hr after meiotic induction, after which they are resolved (Cromie et al. 2006). The frequencies of intersister and interhomolog joint molecules, measured 4.5 hr after meiotic induction, were similar in wt and the rqh1 mutant (Figure 6C). In particular, there was no substantial increase in intersister species at the expense of interhomolog joint molecules.
Figure 6.—
The rqh1Δ mutation does not affect the frequency of intersister vs. interhomolog joint molecules at mbs1 but does substantially reduce the frequency of crossovers at mbs1. (A) The mbs1 region of chromosome I from diploids heterozygous for PmlI and XbaI restriction sites, flanking the mbs1 hotspot. PvuII, PmlI (“L”), and XbaI (“R”) digestion and probing as shown reveal two parental (9.2- and 6.8-kb) and two recombinant (11.2- and 4.8-kb) fragments. Solid arrows indicate DSB sites. (B) Predicted migration during 2-D gel electrophoresis of mbs1-probed DNA digested with PvuII, PmlI, and XbaI (Figure 6A). X-shaped molecules arise during recombination and can be distinguished on the basis of their sizes. Intersister joint molecules (IS) arising from either parent (P1 X or P2 X) migrate as spikes, whereas interhomolog JMs (IH X) migrate as two spots connected by an arc and run at a position between the two intersister spikes (Cromie et al. 2006). (C) DNA isolated 5 hr after meiotic induction of strains GP5086 (rqh1+) and GP6489 (rqh1Δ) was analyzed as in Figure 6B. Solid arrowheads indicate X-form JM species. The thin arrow indicates a partial digestion product or a crossover product. The mean intersister (IS) and interhomolog (IH) JM frequencies are indicated. Values are means of at least two independent experiments ± SEM (n = 3, GP5086) or with the range shown (n = 2, GP6489). The GP5086 data were previously published in Cromie et al. (2006). (D) DNA was isolated at the indicated times after meiotic induction of strains GP5086 (rqh1+) and GP6489 (rqh1Δ) and analyzed by gel electrophoresis and Southern blotting with digestion and probing at mbs1 as in Figure 6A. Asterisks indicate cross-hybridizing DNA not specific to meiosis. The mean crossover frequency among DNA species at 6 hr was calculated as 2 × R2/total. Values are means of at least two independent experiments ± SEM (n = 3, GP5086) or with the range shown (n = 2, GP6489). The GP5086 data were previously published in Cromie et al. (2006).
The crossover products of recombination can be measured physically at mbs1 using the same restriction-site markers as above (Figure 6, A and D; Cromie et al. 2006). In contrast to joint molecules, the final level of crossovers at mbs1 was reduced substantially in the rqh1 mutant, compared to wt (Figure 6D), consistent with the genetic analyses of crossover frequencies in other regions (Figure 1). Therefore, it appears that the reduction in meiotic crossover frequencies seen in an rqh1 mutant is not explained by a change in partner choice, with more intersister events in the rqh1 mutant.
DISCUSSION
In this study we examined the effect on meiotic recombination of null mutations affecting the helicases Rqh1 and Srs2. We observed that the srs2 mutation has at most a mild effect on recombination. In contrast, the rqh1 mutation substantially impaired both gene conversion and crossing over (Figure 1), whereas mutations in the corresponding gene in budding yeast, SGS1, slightly increase meiotic recombination frequencies (Rockmill et al. 2003; Jessop et al. 2006; Oh et al. 2007). Despite the substantial recombination defect in the rqh1 mutant, the maximal level of meiotic DSBs is not reduced, and the breaks are successfully repaired (Figure 4). Spore viability is only moderately reduced in the rqh1 mutant (Figure 3), consistent with a chromosome segregation defect caused simply by the reduced level of meiotic crossovers. Both a physical assay (Figure 6) and a genetic assay for noninterhomolog recombination (Figure 5) argue against one possible explanation for these phenotypes, that meiotic DSB repair is redirected from interhomolog to intersister recombination in the rqh1 mutant. Although the molecular basis of the meiotic recombination defect in rqh1 mutants remains undetermined, below we suggest one possibility, that Rqh1 extends hybrid DNA in joint DNA molecules and favors their resolution to crossovers rather than noncrossovers. What is clear, however, is that Rqh1 in fission yeast plays a different role in meiotic recombination than does Sgs1 in budding yeast. Below, we discuss the implications of these findings.
Role of Rqh1 in meiotic recombination in fission yeast:
Starting with the observation that meiotic crossover frequencies were reduced in an rqh1 mutant, we set out to identify the meiotic role of the Rqh1 protein using a range of recombination and repair assays. The results of these assays argued against any of the possible roles for Rqh1 that we tested directly. This leaves two possibilities: either Rqh1 has a role different from any we have tested or our assays do not properly test one of these roles for Rqh1.
We think our gene conversion, crossover, and spore-viability assays are unlikely to be misleading. Therefore, we are confident that gene conversion and crossover levels are reduced in the rqh1 mutant, and that this phenotype cannot be explained by a failure to repair meiotic DSBs.
On the basis of physical analysis of meiotic DNA, we also conclude that the frequency of meiotic DSB formation and the frequency of intersister and interhomolog joint-molecule formation are not changed in the rqh1 mutant. However, these conclusions are based on measuring the levels of transient intermediates, so that comparisons of frequencies in rqh1+ and rqh1Δ could be misleading, but only if recombination events occurring in the two genetic backgrounds have fundamentally different properties. Specifically, if the frequency of DSBs is reduced in the rqh1 mutant, their life span must be increased to obtain approximately the same transient level as that seen in rqh1+. Similarly, if the rqh1 mutant increases the formation of intersister joint molecules at the expense of interhomolog joint molecules, these effects must both be counteracted by changes in life spans. We prefer the simpler interpretation that DSBs and interhomolog joint molecules form at normal frequency in the rqh1 mutant.
The line of argument presented above suggests that the rqh1 mutation causes a defect in a step in the normal recombination pathway that occurs after interhomolog joint-molecule formation. We can envisage two possibilities that are consistent with, although not directly tested by, our data.
First, Rqh1 could be required to stabilize interhomolog joint molecules, perhaps through extension of regions of hybrid DNA using its helicase activity. In the absence of Rqh1 most of these interhomolog joint molecules would fall apart. Final repair might then occur, in a second step, against the sister chromatid. This step would be unique to the rqh1 mutant. These putative secondary events might not be detected by our 2-D gel assay if, for example, they arise via a SDSA pathway, whose characteristic physical intermediates are not detectable by current methods. In this scheme, a minority of interhomolog joint molecules in the rqh1 mutant would progress to give crossovers with a normal frequency of association with gene conversion (Figure 2).
Second, Rqh1 could be required to promote interhomolog crossovers vs. noncrossovers. This would predict that gene conversion frequencies would be unaffected in an rqh1 mutant while the association of crossovers with gene conversion would be reduced, in both cases contrary to our observations. However, gene conversions occur only in regions of heteroduplex DNA. Therefore, Rqh1 promotion of interhomolog crossovers rather than noncrossovers could be reconciled with our data if the additional noncrossovers in the rqh1 mutant are associated with very short regions of hybrid DNA. Again, this would suggest the role of Rqh1 is to extend regions of hybrid DNA, using its helicase activity. In this scheme, a minority of interhomolog joint molecules in the rqh1 mutant would still progress to give extensive heteroduplex DNA, with normal levels of gene conversion and associated crossovers.
Differences between the phenotypes of rqh1 and sgs1 mutants:
Why are the meiotic phenotypes of fission and budding yeast mutants lacking RecQ homologs so different? Budding yeast Sgs1 and fission yeast Rqh1, in common with human BLM, have the same domain structure, and sgs1 and rqh1 mutants share many mitotic phenotypes, including sensitivity to DNA damaging agents, synthetic lethality with srs2 or mus81 mutations, and increased frequencies of mitotic recombination (Stewart et al. 1997; Boddy et al. 2000; Gangloff et al. 2000; Mullen et al. 2000, 2001; Maftahi et al. 2002). The amino acid sequences of the two proteins are 29% identical. However, during meiosis, Rqh1 appears to have a prorecombination role while Sgs1 has an antirecombination role.
Recent evidence indicates that an important meiotic role for Sgs1 is an interaction with the ZMM group of proteins whose prorecombination activity antagonizes an antirecombination activity of Sgs1 (Jessop et al. 2006; Oh et al. 2007). The ZMM proteins are specifically required for the formation of the class of budding-yeast crossovers that are subject to interference, the process by which one crossover discourages the occurrence of further crossovers nearby (reviewed by Cromie and Smith 2007; Lynn et al. 2007). zmm mutants are substantially recombination defective, but this effect is largely suppressed by a further mutation affecting SGS1 (Jessop et al. 2006; Oh et al. 2007).
The ZMM proteins do not seem to be present in fission yeast, which also lacks the class of crossovers that are subject to interference (reviewed by Cromie and Smith 2007). Therefore, it is perhaps not surprising that rqh1 mutants lack those phenotypes of sgs1 mutants that are associated with the interplay between Sgs1 and the ZMM proteins. However, it is more surprising that Rqh1 appears to have an additional and completely different role in fission yeast meiosis, the promotion of homologous recombination.
These observations imply that Sgs1 has a meiotic role that Rqh1 lacks and vice versa. The budding yeast-specific role of Sgs1 appears to be related to features of that organism (the ZMM proteins and crossover interference) that are absent from fission yeast meiosis. It is tempting to speculate that the meiotic role of Rqh1 is similarly related to a fission yeast-specific process. One possibility is that Rqh1 operates on fission yeast-specific DNA intermediates. This is consistent with the recent observation that the majority of joint molecules in fission yeast meiosis contain single Holliday junctions, rather than the predominant double Holliday junctions found in budding yeast (Cromie et al. 2006). Perhaps Rqh1 and Sgs1 carry out the same mitotic function, but, because the meiotic function of Sgs1 is unnecessary in fission yeast, Rqh1 has been recruited to process fission-yeast meiotic-recombination intermediates that do not have equivalents in budding yeast.
Acknowledgments
We thank Greg Freyer for S. pombe strains; Jeff Virgin, Jennifer Young, and Chad Ellermeier for unpublished results on rqh1 mutants; and Susan Amundsen, Luther Davis, and Jennifer Geelhood for critical reading of the manuscript. This work was supported by research grants GM-031693 and GM-032194 from the National Institutes of Health to G.R.S.
References
- Ahmad, F., and E. Stewart, 2005. The N-terminal region of the Schizosaccharomyces pombe RecQ helicase, Rqh1p, physically interacts with Topoisomerase III and is required for Rqh1p function. Mol. Genet. Genomics 273 102–114. [DOI] [PubMed] [Google Scholar]
- Bennett, R. J., J. L. Keck and J. C. Wang, 1999. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S. cerevisiae. J. Mol. Biol. 289 235–248. [DOI] [PubMed] [Google Scholar]
- Boddy, M. N., A. Lopez-Girona, P. Shanahan, H. Interthal, W. D. Heyer et al., 2000. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 20 8758–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti, R. S., S. Schonberg and J. German, 1974. A many-fold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc. Natl. Acad. Sci. USA 71 4508–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb, J. A., and L. Bjergbaek, 2006. RecQ helicases: lessons from model organisms. Nucleic Acids Res. 34 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie, G. A., and G. R. Smith, 2008. Meiotic recombination in Schizosaccharomyces pombe: a paradigm for genetic and molecular analysis, in Recombination and Meiosis, edited by R. Egel. Springer-Verlag, Berlin (in press). [DOI] [PMC free article] [PubMed]
- Cromie, G. A., and G. R. Smith, 2007. Branching out: meiotic recombination and its regulation. Trends Cell Biol. 17 448–455. [DOI] [PubMed] [Google Scholar]
- Cromie, G. A., C. A. Rubio, R. W. Hyppa and G. R. Smith, 2005. A natural meiotic DNA break site in Schizosaccharomyces pombe is a hotspot of gene conversion, highly associated with crossing over. Genetics 169 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie, G. A., R. W. Hyppa, A. F. Taylor, K. Zakharyevich, N. Hunter et al., 2006. Single Holliday junctions are intermediates of meiotic recombination. Cell 127 1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2001. Meiotic recombination and chromosome segregation in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 98 8395–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2003. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163 857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2006. The meiotic bouquet promotes homolog interactions and restricts ectopic recombination in Schizosaccharomyces pombe. Genetics 174 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVeaux, L. C., N. A. Hoagland and G. R. Smith, 1992. Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics 130 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe, C. L., and M. C. Whitby, 2004. The involvement of Srs2 in post-replication repair and homologous recombination in fission yeast. Nucleic Acids Res. 32 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe, C. L., J. Dixon, F. Osman and M. C. Whitby, 2000. Partial suppression of the fission yeast rqh1(−) phenotype by expression of a bacterial Holliday junction resolvase. EMBO J. 19 2751–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier, C., H. Schmidt and G. R. Smith, 2004. Swi5 acts in meiotic DNA joint molecule formation in Schizosaccharomyces pombe. Genetics 168 1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, N. A., J. Grodent, Z. Ye, J. Straughend, J. Lennon et al., 1995. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83 655–666. [DOI] [PubMed] [Google Scholar]
- Gangloff, S., C. Soustelle and F. Fabre, 2000. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat. Genet. 25 192–194. [DOI] [PubMed] [Google Scholar]
- Grimm, C., J. Bahler and J. Kohli, 1994. M26 recombinational hotspot and physical conversion tract analysis in the ade6 gene of Schizosaccharomyces pombe. Genetics 136 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz, H., 1971. Site specific induction of gene conversion in Schizosaccharomyces pombe. Genetics 69 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygax, A., and P. Thuriaux, 1984. A revised chromosome map of the fission yeast Schizossacharomyces pombe. Curr. Genet. 8 85–92. [DOI] [PubMed] [Google Scholar]
- Haldane, J. B. S., 1919. The combination of linkage values, and the calculation of distance between the loci of linked factors. J. Genet. 8 299–309. [Google Scholar]
- Hanada, K., and I. D. Hickson, 2007. Molecular genetics of RecQ helicase disorders. Cell. Mol. Life Sci. 64 2306–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, J. C., L. D. Cruzata, A. Duvshani, J. Mitsumoto, M. Maftahi et al., 2007. Mus81-Eme1-dependent and -independent crossovers form in mitotic cells during double-strand break repair in Schizosaccharomyces pombe. Mol. Cell. Biol. 27 3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, J. C., S. M. Mense, M. Jalakas, J. Mitsumoto and G. A. Freyer, 2006. Rqh1 blocks recombination between sister chromatids during double strand break repair, independent of its helicase activity. Proc. Natl. Acad. Sci. USA 103 5875–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyppa, R. W., and G. R. Smith, 2008. Using Schizosaccharomyces pombe meiosis to analyze DNA recombination intermediates, in Meiosis, edited by S. Keeney. Humana Press, Clifton, NJ (in press). [DOI] [PMC free article] [PubMed]
- Iino, Y., and M. Yamamoto, 1985. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 82 2447–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira, G., A. Malkova, G. Liberi, M. Foiani and J. E. Haber, 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop, L., B. Rockmill, G. S. Roeder and M. Lichten, 2006. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of Sgs1. PLoS Genet. 2 e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao, S., A. Shimamoto, M. Goto, R. W. Miller, W. A. Smithson et al., 1999. Mutations in RECQL4 cause a subset of cases of Rothmund–Thomson syndrome. Nat. Genet. 22 82–84. [DOI] [PubMed] [Google Scholar]
- Lawrence, C. W., and R. B. Christensen, 1979. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J. Bacteriol. 139 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberi, G., G. Maffioletti, C. Lucca, I. Chiolo, A. Baryshnikova et al., 2005. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and G. R. Smith, 1994. Transient, meiosis-induced expression of the rec6 and rec12 genes of Schizosaccharomyces pombe. Genetics 136 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, A., R. Soucek and G. V. Börner, 2007. ZMM proteins during meiosis: crossover artists at work. Chromosome Res. 15 591–605. [DOI] [PubMed] [Google Scholar]
- Maftahi, M., J. C. Hope, L. Delgado-Cruzata, C. S. Han and G. A. Freyer, 2002. The severe slow growth of Δsrs2 Δrqh1 in Schizosaccharomyces pombe is suppressed by loss of recombination and checkpoint genes. Nucleic Acids Res. 30 4781–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri, H. W., T. J. Craig and A. Morgan, 2002. SGS1 is a multicopy suppressor of srs2: functional overlap between DNA helicases. Nucleic Acids Res. 30 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194 795–823. [DOI] [PubMed] [Google Scholar]
- Mullen, J. R., V. Kaliraman and S. J. Brill, 2000. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics 154 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, J. R., V. Kaliraman, S. S. Ibrahim and S. J. Brill, 2001. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, S. D., J. P. Lao, P. Y. Hwang, A. F. Taylor, G. R. Smith et al., 2007. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino, F., and H. L. Klein, 1992. Analysis of mitotic and meiotic defects in Saccharomyces cerevisiae SRS2 DNA helicase mutants. Genetics 132 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli, A. S., and G. R. Smith, 1989. Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics 123 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranam, K. L., and P. J. Blackshear, 1994. Cloning and characterization of RECQL, a potential human homologue of the Escherichia coli DNA helicase RecQ. J. Biol. Chem. 269 29838–29845. [PubMed] [Google Scholar]
- Rockmill, B., J. C. Fung, S. S. Branda and G. S. Roeder, 2003. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr. Biol. 13 1954–1962. [DOI] [PubMed] [Google Scholar]
- Rong, L., F. Palladino, A. Aguilera and H. L. Klein, 1991. The hyper-gene conversion hpr5–1 mutation of Saccharomyces cerevisiae is an allele of the SRS2/RADH gene. Genetics 127 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchert, P., and J. Kohli, 1988. The ade6–M26 mutation of Schizosaccharomyces pombe increases the frequency of crossing over. Genetics 119 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. R., 2008. Genetic analysis of meiotic recombination in Schizosaccharomyces pombe, in Meiosis, edited by S. Keeney, Humana Press, Clifton, NJ (in press). [DOI] [PMC free article] [PubMed]
- Stewart, E., C. R. Chapman, F. Al-Khodairy, A. M. Carr and T. Enoch, 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16 2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli, M., M. Shayeghi, A. Nasim and F. Z. Watts, 1995. Cloning and characterisation of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res. 23 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. W., A. Goodwin, I. D. Hickson and C. J. Norbury, 2001. Involvement of Schizosaccharomyces pombe Srs2 in cellular responses to DNA damage. Nucleic Acids Res. 29 2963–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt, P. M., I. D. Hickson, R. H. Borts and E. J. Louis, 1996. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics 144 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, E. B., 1927. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22 209–212. [Google Scholar]
- Wu, L., C. Z. Bachrati, J. Ou, C. Xu, J. Yin et al., 2006. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc. Natl. Acad. Sci. USA 103 4068–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. A., R. W. Schreckhise, W. W. Steiner and G. R. Smith, 2002. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell 9 253–263. [DOI] [PubMed] [Google Scholar]
- Young, J. A., R. W. Hyppa and G. R. Smith, 2004. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics 167 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. E., J. Oshima, Y. H. Fu, E. M. Wijsman, F. Hisama et al., 1996. Positional cloning of the Werner's syndrome gene. Science 272 258–262. [DOI] [PubMed] [Google Scholar]
- Zahn-Zabal, M., E. Lehmann and J. Kohli, 1995. Hot spots of recombination in fission yeast: inactivation of the M26 hot spot by deletion of the ade6 promoter and the novel hotspot ura4-aim. Genetics 140 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]