Abstract
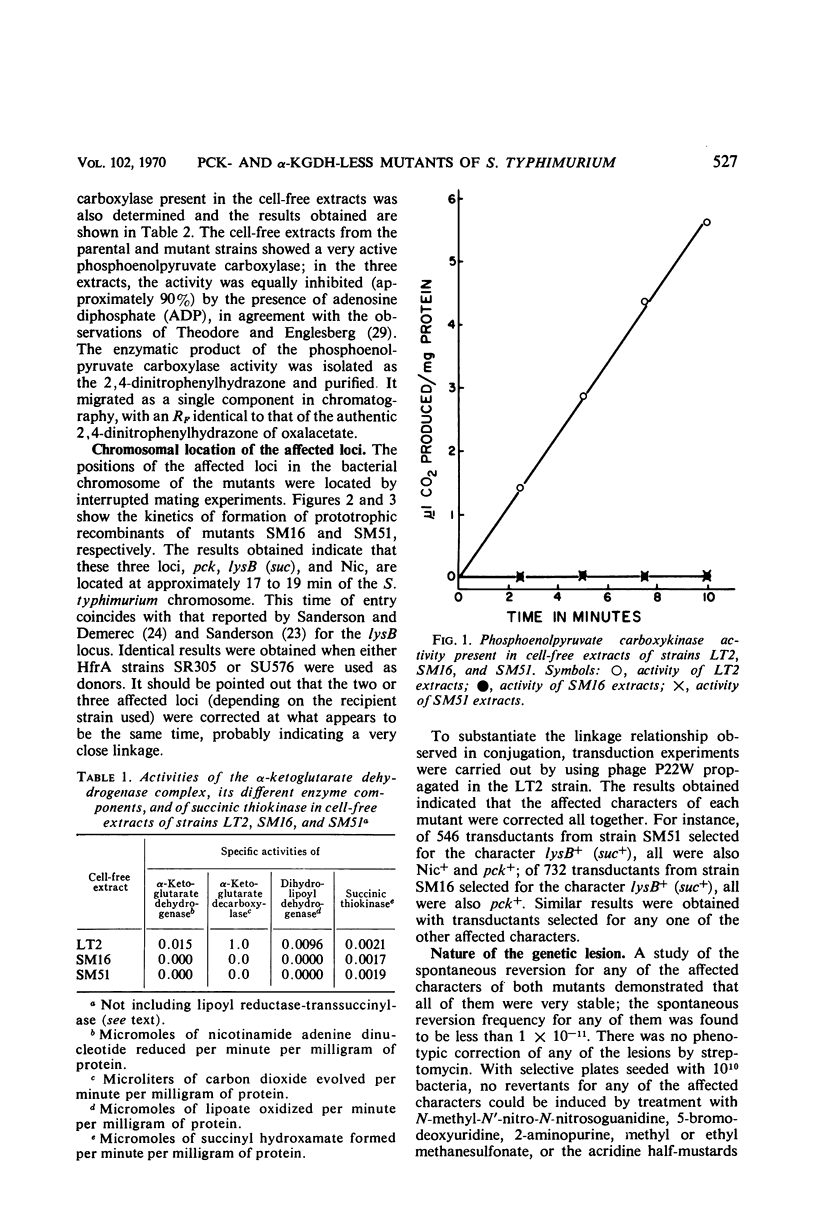
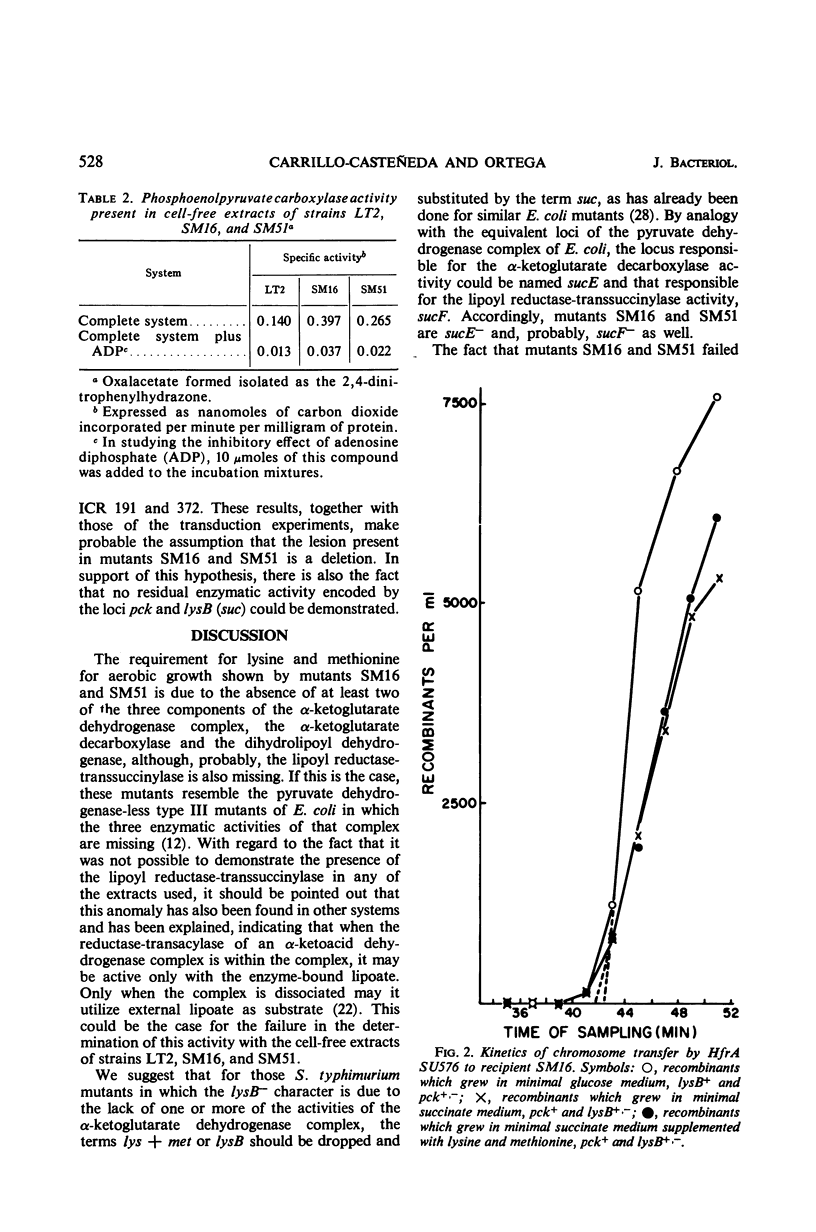
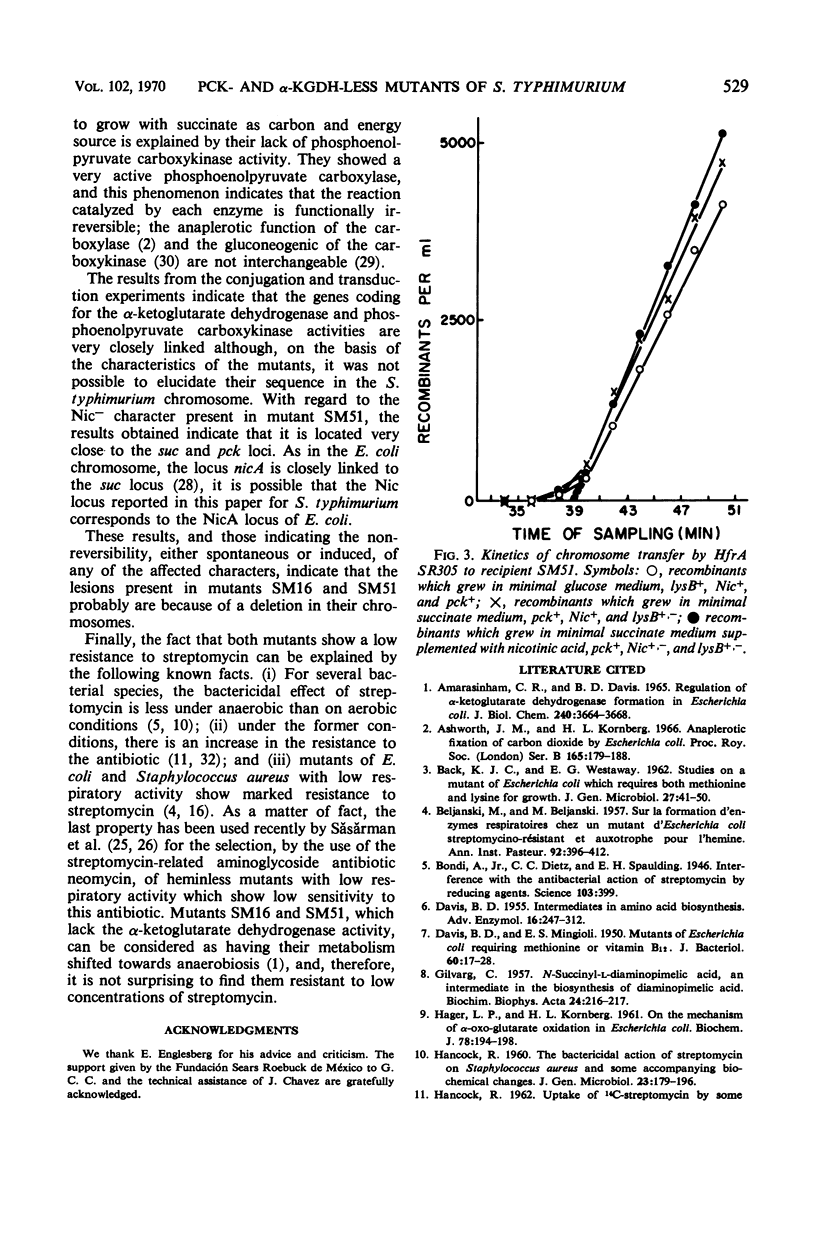
Two auxotrophic mutants (SM16 and SM51) of Salmonella typhimurium, which for aerobic growth, with hexoses as carbon source, required lysine and methionine (SM51 required also nicotinic acid), were isolated and characterized. The requirement for the amino acids disappeared in anaerobiosis. Neither lipoate nor 4-hydroxybenzoate was effective in supporting aerobic growth of the mutants. The lysine and methionine requirement for aerobic growth was due to the absence in the mutants of the enzymatic activities of the α-ketoglutarate dehydrogenase complex. The mutants could not use succinate as carbon source even after enrichment of the growth medium with acid-hydrolyzed casein and yeast extract. No phosphoenolpyruvate carboxykinase activity was found in the mutants, a phenomenon which explained their inability to use succinate. By interrupted conjugation and by transduction experiments, the positions of the three affected loci, pck, suc, and Nic, were located at approximately 17 to 19 min of the S. typhimurium chromosome; they were found to be closely linked. From different criteria, it appears as if the genetic lesions present in both mutants are due to deletion of a small chromosome fragment.
Full text
PDF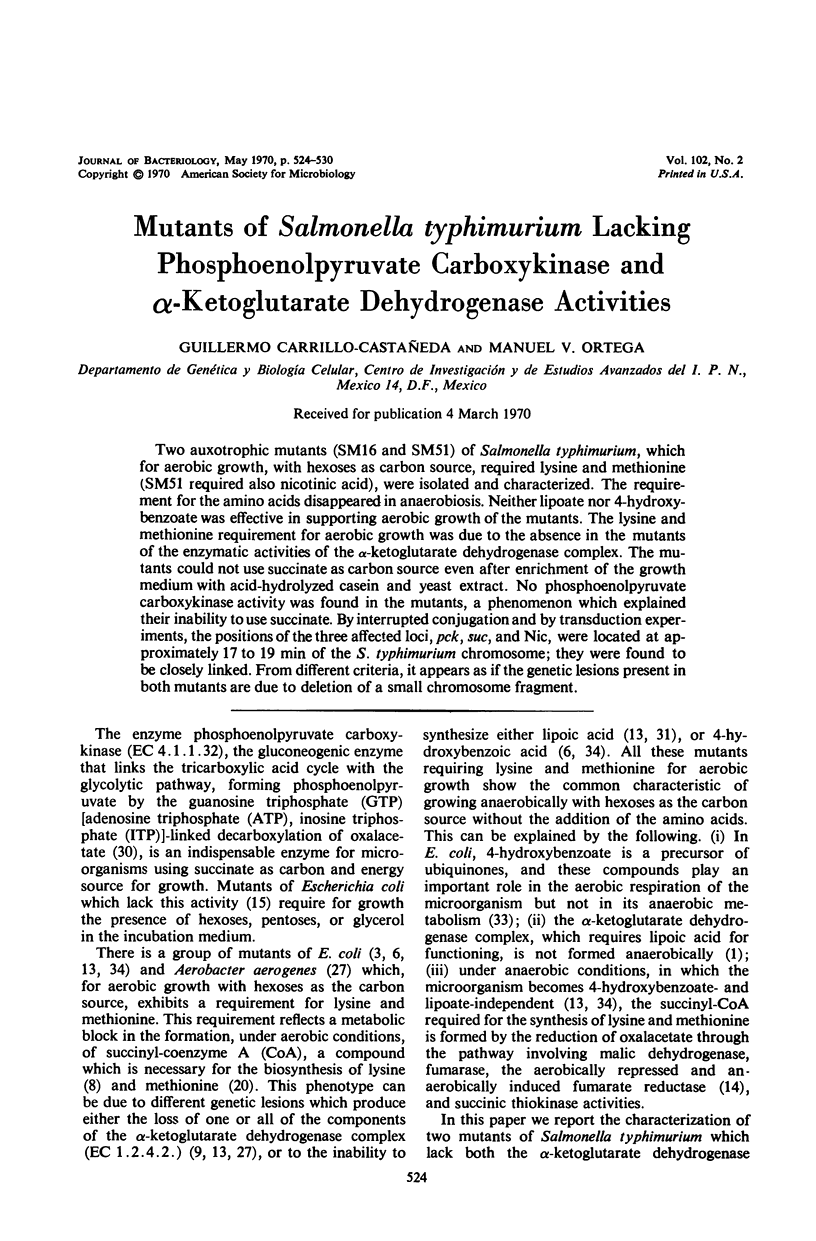



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- BACK K. J., WESTAWAY E. G. Studies on a mutant strain of Escherichia coli which requires both methionine and lysine for growth. J Gen Microbiol. 1962 Jan;27:41–50. doi: 10.1099/00221287-27-1-41. [DOI] [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- Bondi A., Jr, Dietz C. C., Spaulding E. H. Interference With the Antibacterial Action of Streptomycin by Reducing Agents. Science. 1946 Mar 29;103(2674):399–401. doi: 10.1126/science.103.2674.399. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Intermediates in amino acid biosynthesis. Adv Enzymol Relat Subj Biochem. 1955;16:247–312. doi: 10.1002/9780470122617.ch5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILVARG C. N-succinyl-L-diamino-pimelic acid, an intermediate in the biosynthesis of diaminopimelic acid. Biochim Biophys Acta. 1957 Apr;24(1):216–217. doi: 10.1016/0006-3002(57)90176-2. [DOI] [PubMed] [Google Scholar]
- HAGER L. P., KORNBERG H. L. On the mechanism of alpha-oxoglutarate oxidation in Escherichia coli. Biochem J. 1961 Jan;78:194–198. doi: 10.1042/bj0780194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK R. The bactericidal action of streptomycin on Staphylococcus aureus and some accompanying biochemical changes. J Gen Microbiol. 1960 Aug;23:179–196. doi: 10.1099/00221287-23-1-179. [DOI] [PubMed] [Google Scholar]
- HANCOCK R. Uptake of 14C-streptomycin by some microorganisms and its relation to their streptomycin sensitivity. J Gen Microbiol. 1962 Jul;28:493–501. doi: 10.1099/00221287-28-3-493. [DOI] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Jones R. G., Lascelles J. The relationship of 4-hydroxybenzoic acid to lysine and methionine formation in Escherichia coli. Biochem J. 1967 Jun;103(3):709–713. doi: 10.1042/bj1030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. G. Ubiquinone deficiency in an auxotroph of Escherichia coli requiring 4-hydroxybenzoic acid. Biochem J. 1967 Jun;103(3):714–719. doi: 10.1042/bj1030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUN E., GARCIA-HERNANDEZ M. Identification and quantitative determination of keto acids by paper chromatography. Biochim Biophys Acta. 1957 Jan;23(1):181–186. doi: 10.1016/0006-3002(57)90301-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. O-SUCCINYLHOMOSERINE AS AN INTERMEDIATE IN THE SYNTHESIS OF CYSTATHIONINE BY ESCHERICHIA COLI. J Gen Microbiol. 1964 Sep;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., SEARLS R. L. alpha-Ketoglutaric dehydrogenase. VI. Reversible oxidation of dihydrothioctamide by diphosphopyridine nucleotide. J Biol Chem. 1959 Jan;234(1):178–182. [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Horodniceanu T. Locus determining normal colony formation on the chromosome of Escherichia coli K-12. J Bacteriol. 1967 Oct;94(4):1268–1269. doi: 10.1128/jb.94.4.1268-1269.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODORE T. S., ENGLESBERG E. MUTANT OF SALMONELLA TYPHIMURIUM DEFICIENT IN THE CARBON DIOXIDE-FIXING ENZYME PHOSPHOENOLPYRUVIC CARBOXYLASE. J Bacteriol. 1964 Oct;88:946–955. doi: 10.1128/jb.88.4.946-955.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954 Apr;207(2):821–841. [PubMed] [Google Scholar]
- WILLIAMSON G. M. Dihydrostreptomycin and anaerobiosis; indirect evidence for two sites of action of dihydrostreptomycin. J Gen Microbiol. 1958 Dec;19(3):584–591. doi: 10.1099/00221287-19-3-584. [DOI] [PubMed] [Google Scholar]


