Abstract
In recent years studies of the effectiveness of different typhoid vaccines have been sponsored by the World Health Organization in British Guiana, the USSR, and Yugoslavia. A similarly sponsored study has been made in Poland under the auspices of the Polish Typhoid Committee. In the controlled field trial four types of vaccine were used: (1) bacterial acetone-killed and -dried (vaccines K and P), (2) bacterial formol-killed phenol-preserved (vaccine N), (3) Westphal's endotoxin adsorbed on aluminium hydroxide (vaccine S), and (4) Grasset's vaccine (autolysate of typhoid bacilli adsorbed on aluminium hydroxide; vaccine T). The control vaccine was tetanus toxoid (vaccine O). Laboratory tests were also carried out.
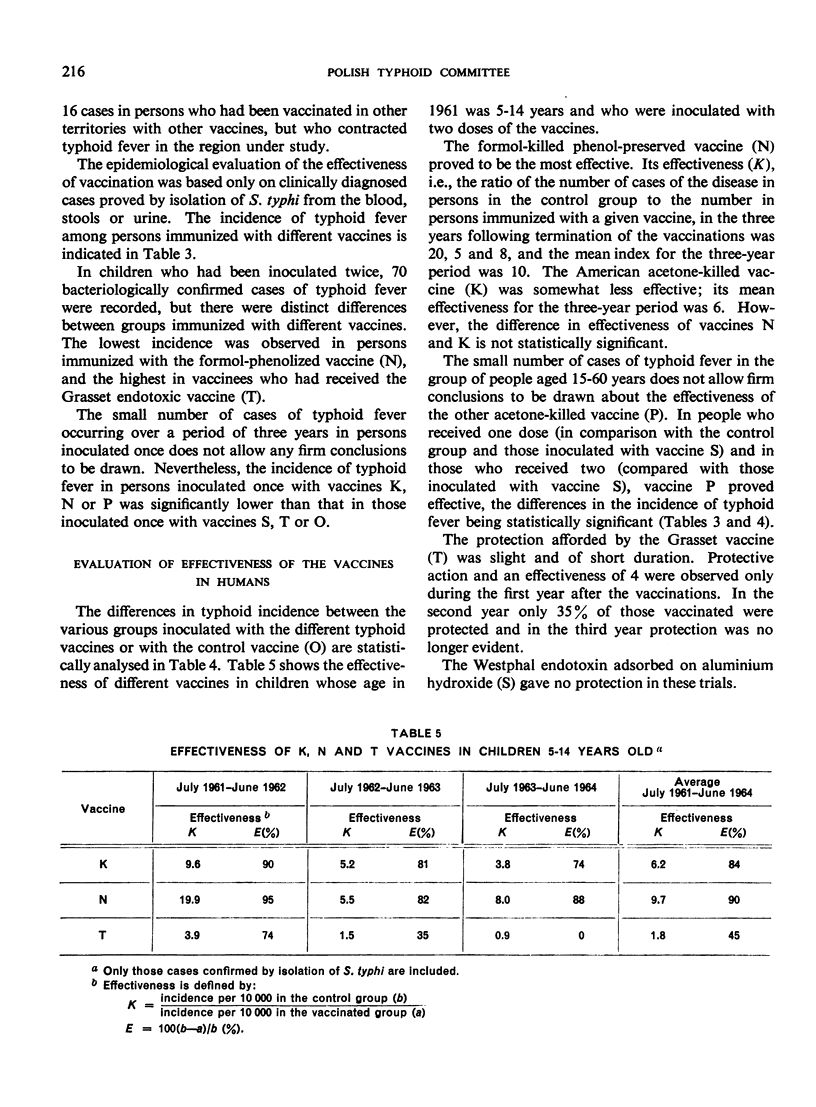
In children aged 5-14 years who received two inoculations, vaccine N was the most effective, followed by K; vaccine T was distinctly less effective. In people aged 15-60 years the small number of typhoid cases made evaluation difficult; however, vaccines N and P inoculated once afforded protection, whereas vaccine S imparted none.
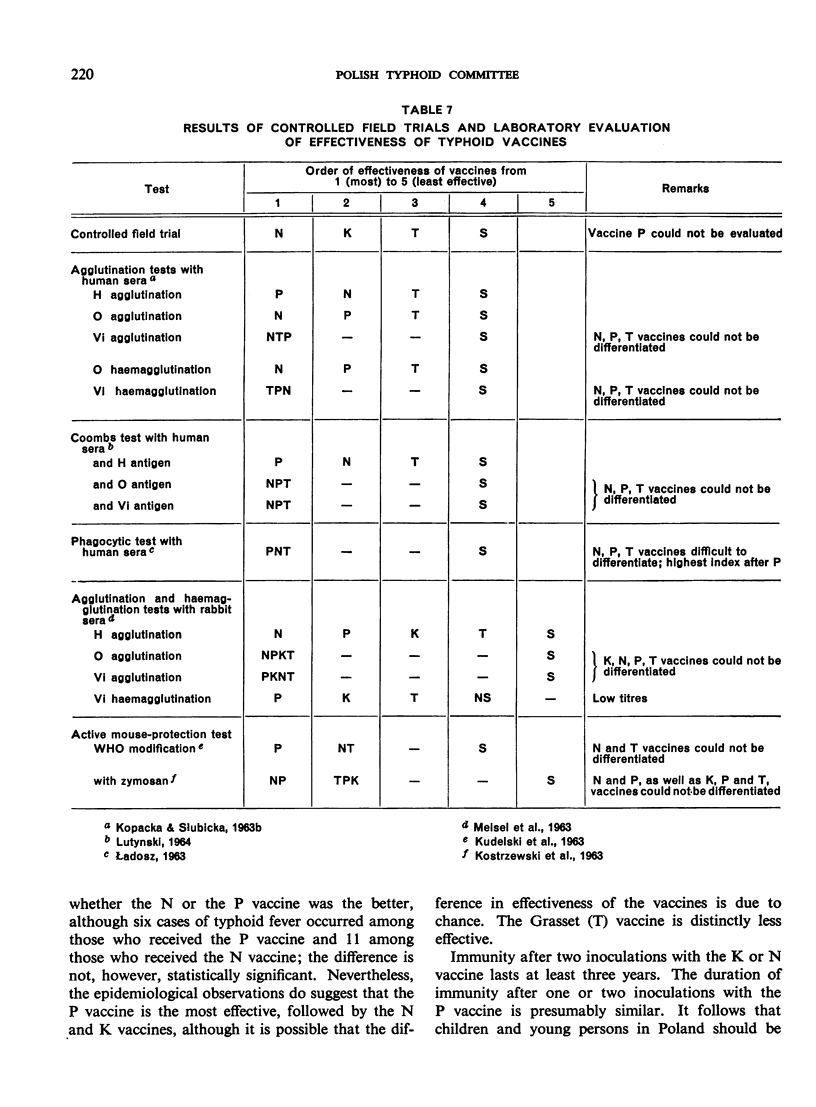
Further studies are desirable on the laboratory testing of typhoid vaccines: at present the H and O agglutination tests with sera of immunized rabbits, combined with an active mouse-protection test, can be recommended, provided that a standard typhoid vaccine is used for comparison.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LUTYNSKI R. OCENA SZCZEPIONEK I SKUTECZNO'SCI SZCZEPIE'N PRZECIW DUROWI BRZUSZNEMU. XIX. SEROLOGICZNE ODCZYNY U LUDZI PO ZASTOSOWANIU R'OZNYCH SZCZEPIONEK PRZECIWDUROWYCH. Przegl Epidemiol. 1964;18:347–353. [PubMed] [Google Scholar]


