Abstract
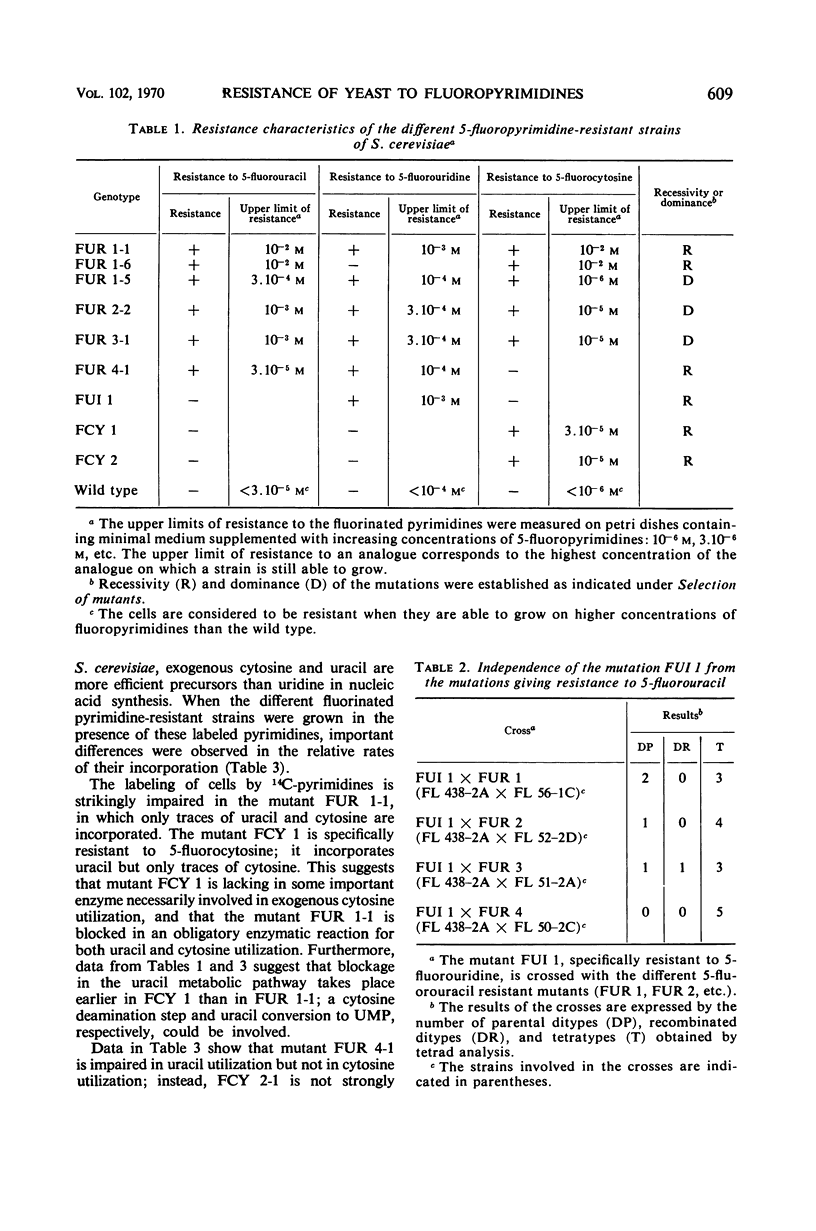
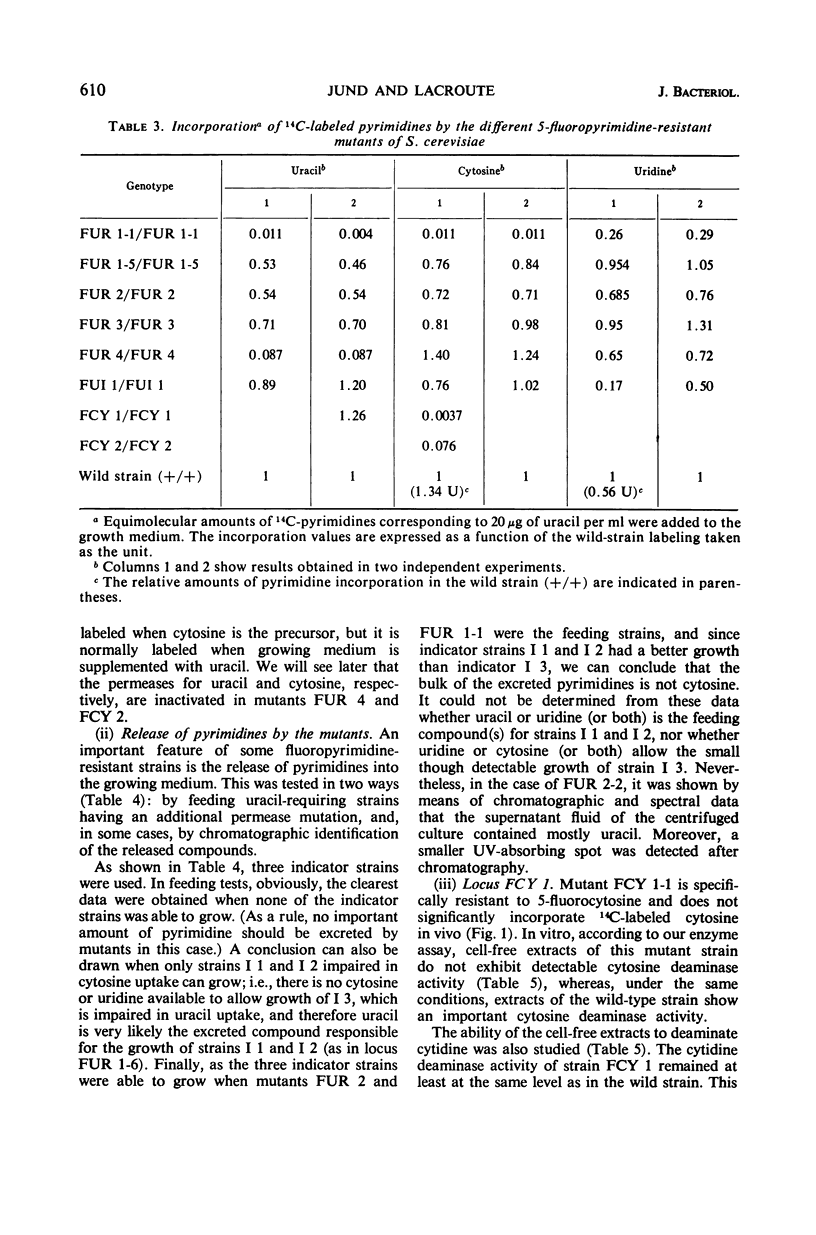
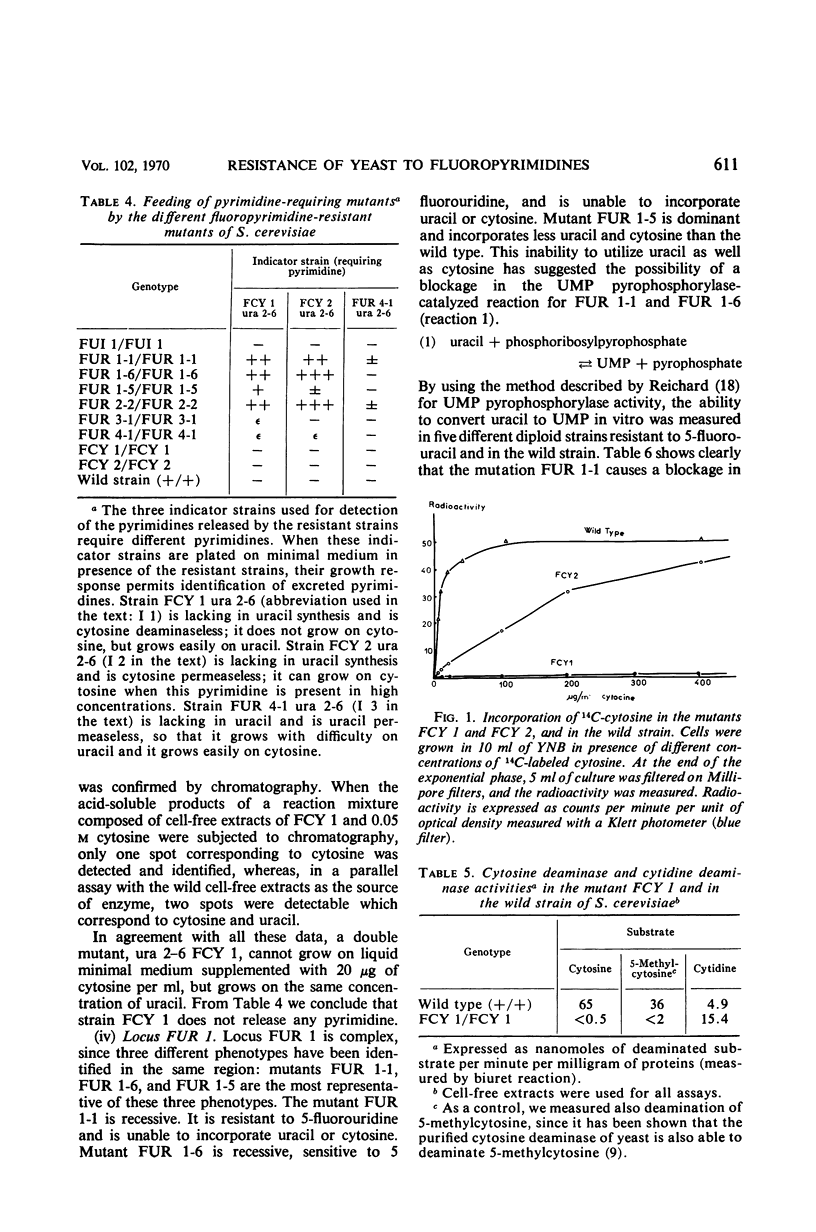
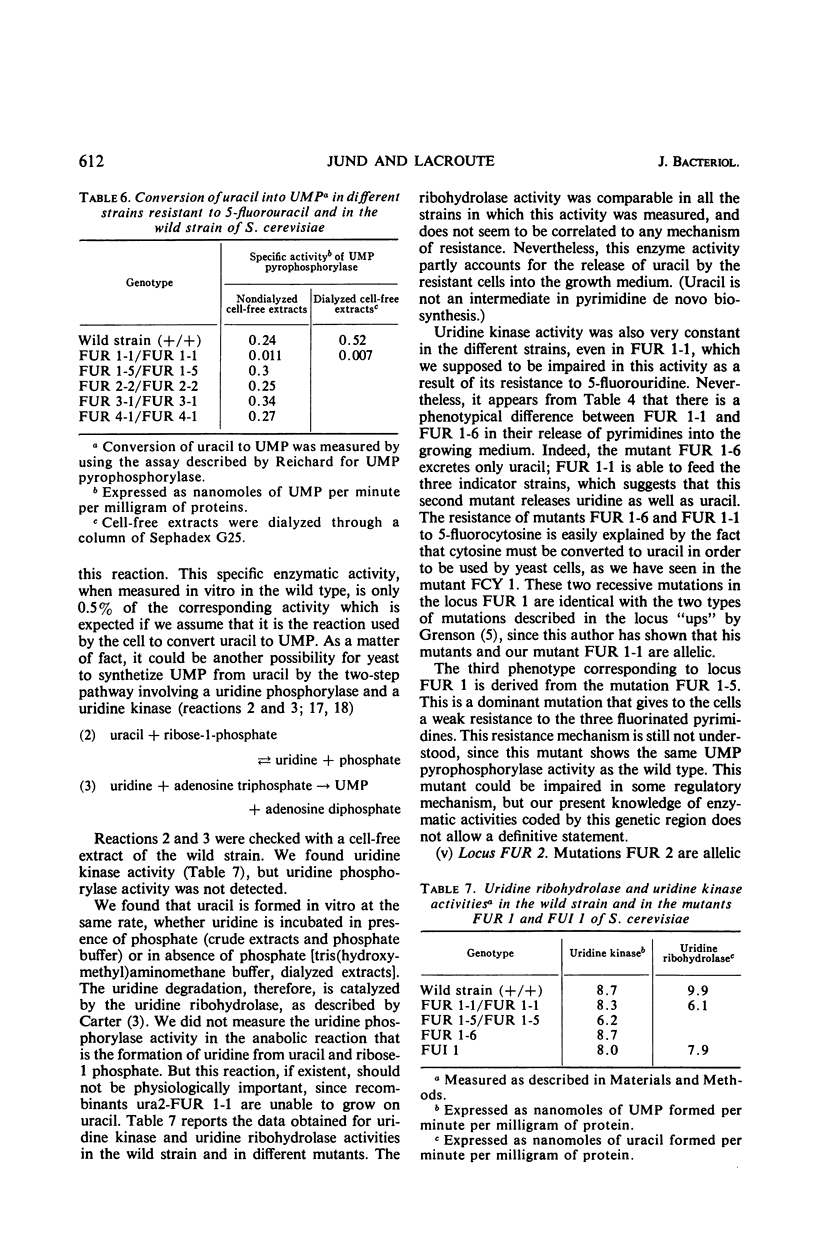
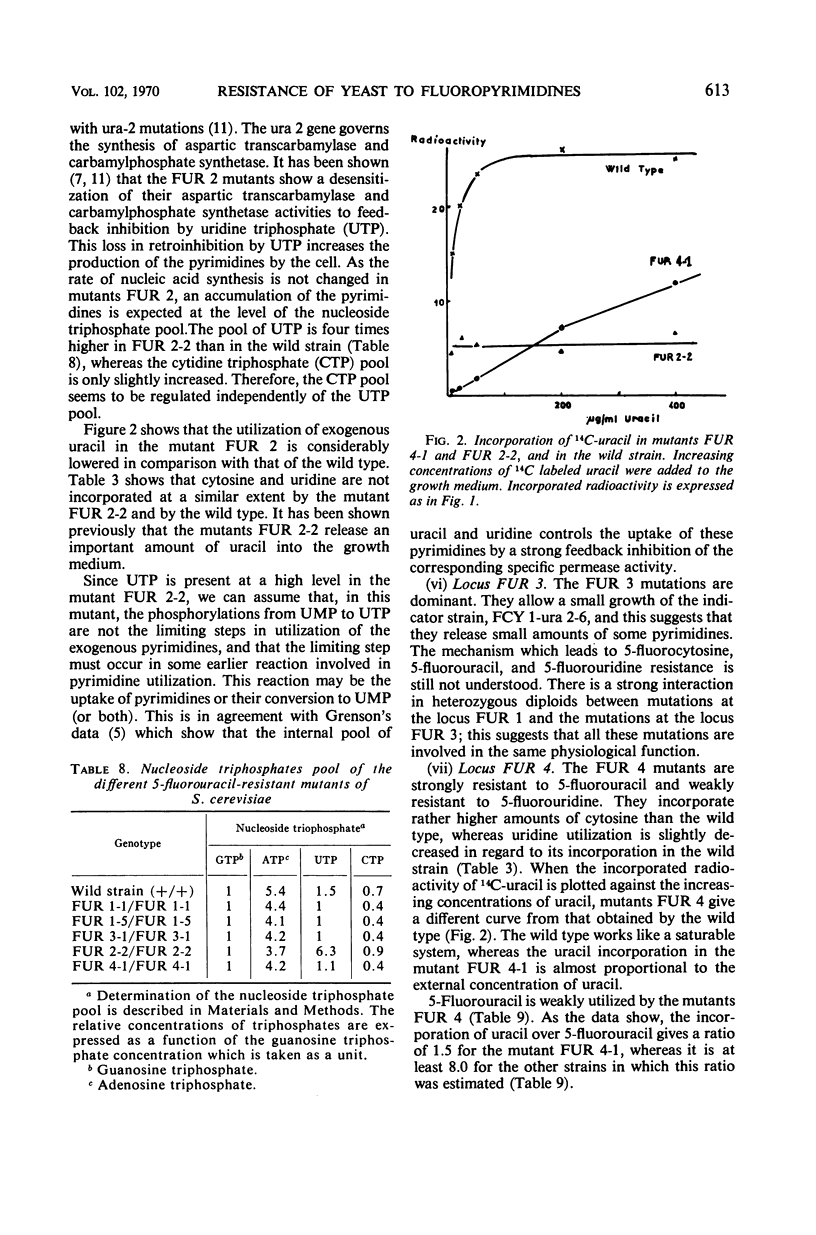
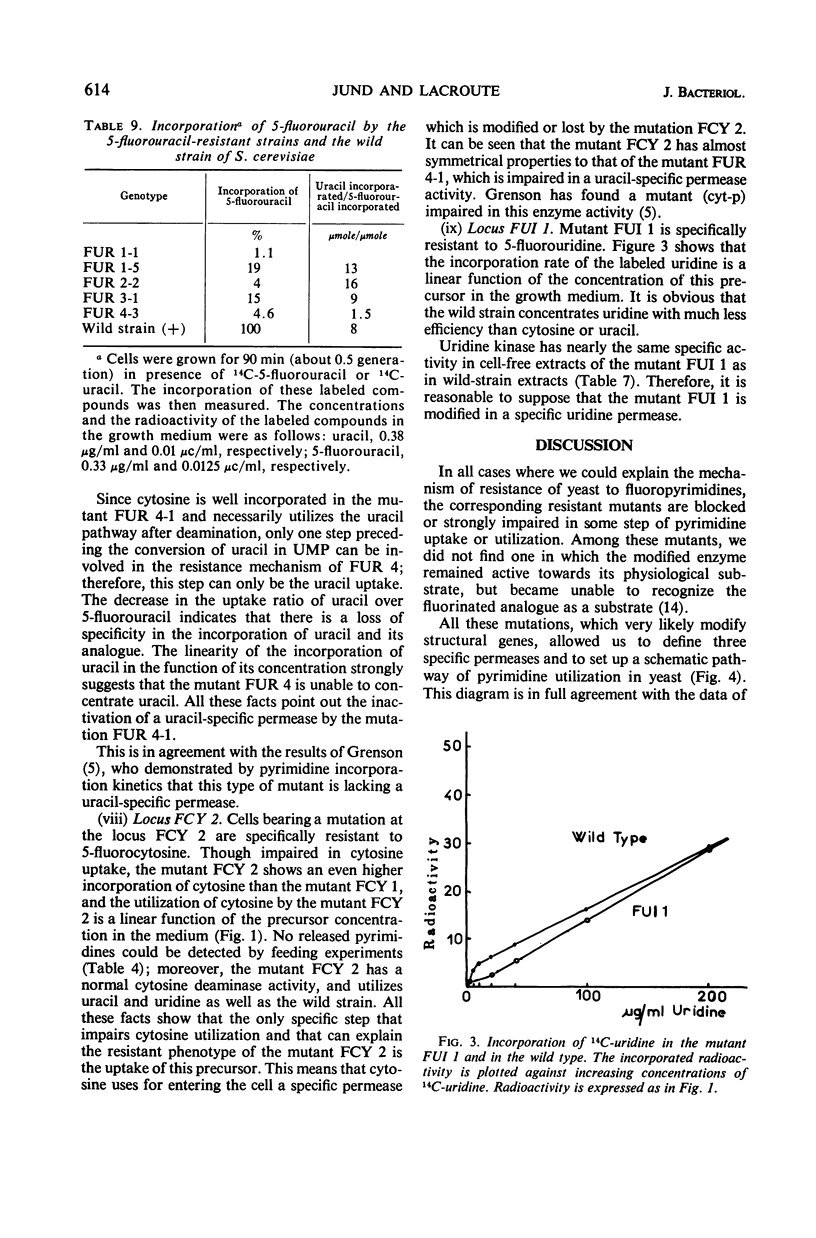
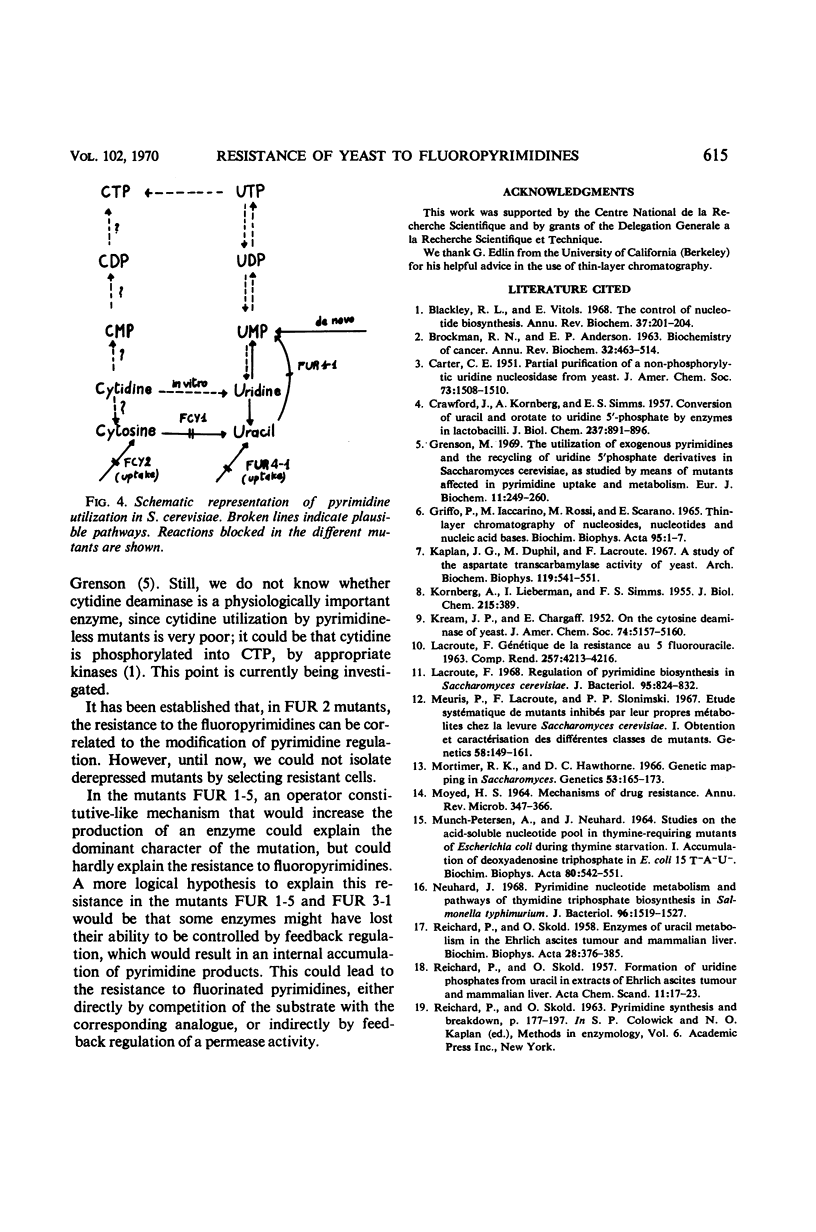
Mutants resistant to 5-fluorouracil, 5-fluorocytosine, and 5-fluorouridine were selected in yeast, and the mechanisms of their resistance were investigated. The investigated mutations map in seven different loci. (i) A mutation at the locus FUI 1 gives specifically resistance to 5-fluorouridine. (ii) Two loci are involved in a specific 5-fluorocytosine resistance: a mutation at locus FCY 1 produces a loss of cytosine deaminase activity; a mutation at locus FCY 2 results in the loss of the activity of a cytosine-specific permease. (iii) A mutation at the locus FUR 4 gives a simultaneous resistance to 5-fluorouracil and to 5-fluorouridine by loss in the activity of the uracil-specific permease. (iv) We found three types of mutants in the locus FUR 1. One is dominant and weakly resistant to 5-fluorouracil, 5-fluorocytosine, and 5-fluorouridine. The two others are recessive and are unable to catalyze one of the steps involved in uracil transformation into uridine 5′-monophosphate; this block-age explains their strong resistance to 5-fluorouracil and 5-fluorocytosine. Of these two mutants, one is resistant to 5-fluorouridine and the other is not. (v) Mutations at locus FUR 2 give resistance to 5-fluorouracil, 5-fluorocytosine, and 5-fluorouridine. These mutations are dominant and lead to a loss in the feedback regulation of the aspartic transcarbamylase activity by uridine triphosphate. (vi) The mutants FUR 3 are resistant to 5-fluorocytosine and 5-fluorouridine. They are dominant and physiologically related to the mutants of the locus FUR 1 but their mechanism of resistance is not understood.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCKMAN R. W., ANDERSON E. P. BIOCHEMISTRY OF CANCER (METABOLIC ASPECTS). Annu Rev Biochem. 1963;32:463–512. doi: 10.1146/annurev.bi.32.070163.002335. [DOI] [PubMed] [Google Scholar]
- Blakley R. L., Vitols E. The control of nucleotide biosynthesis. Annu Rev Biochem. 1968;37:201–224. doi: 10.1146/annurev.bi.37.070168.001221. [DOI] [PubMed] [Google Scholar]
- GRIPPO P., IACCARINO M., ROSSI M., SCARANO E. THIN-LAYER CHROMATOGRAPHY OF NUCLEOTIDES, NUCLEOSIDES AND NUCLEIC ACID BASES. Biochim Biophys Acta. 1965 Jan 11;95:1–7. doi: 10.1016/0005-2787(65)90204-2. [DOI] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., LIEBERMAN I., SIMMS E. S. Enzymatic synthesis and properties of 5-phosphoribosylpyrophosphate. J Biol Chem. 1955 Jul;215(1):389–402. [PubMed] [Google Scholar]
- Kaplan J. G., Duphil M., Lacroute F. A study of the aspartate transcarbamylase activity of yeast. Arch Biochem Biophys. 1967 Mar;119(1):541–551. doi: 10.1016/0003-9861(67)90489-4. [DOI] [PubMed] [Google Scholar]
- LACROUTE F. G'EN'ETIQUE DE LA R'ESISTANCE AU 5-FLUOROURACILE CHEZ LA LEVURE. C R Hebd Seances Acad Sci. 1963 Dec 23;257:4213–4216. [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. BIOCHEMICAL MECHANISMS OF DRUG RESISTANCE. Annu Rev Microbiol. 1964;18:347–366. doi: 10.1146/annurev.mi.18.100164.002023. [DOI] [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., NEUHARD J. STUDIES ON THE ACID-SOLUBLE NUCLEOTIDE POOL IN THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI DURING THYMINE STARVATION. I. ACCUMULATION OF DEOXYADENOSINE TRIPHOSPHATE IN ESCHERICHIA COLI 15 T-A-U-. Biochim Biophys Acta. 1964 Apr 27;80:542–551. doi: 10.1016/0926-6550(64)90298-1. [DOI] [PubMed] [Google Scholar]
- Meuris P., Lacroute F., Slonimski P. P. Etude systematique de mutants inhibes par leurs propres metabolites chez la levure Saccharomyces cerevisiae. I. Obtention et caracterisation des differentes classes de mutants. Genetics. 1967 May;56(1):149–161. doi: 10.1093/genetics/56.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICHARD P., SKOLD O. Enzymes of uracil metabolism in the Ehrlich ascites tumour and mammalian liver. Biochim Biophys Acta. 1958 May;28(2):376–385. doi: 10.1016/0006-3002(58)90485-2. [DOI] [PubMed] [Google Scholar]


