Abstract
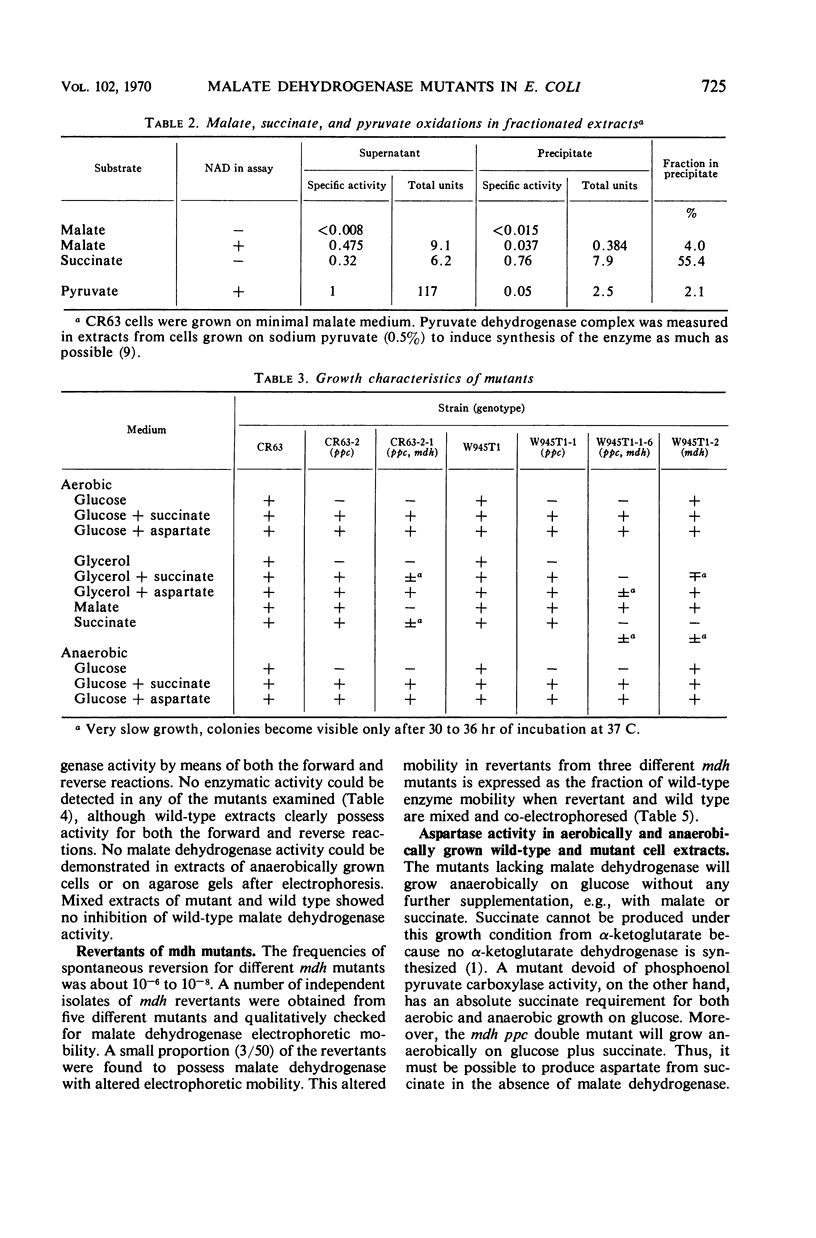
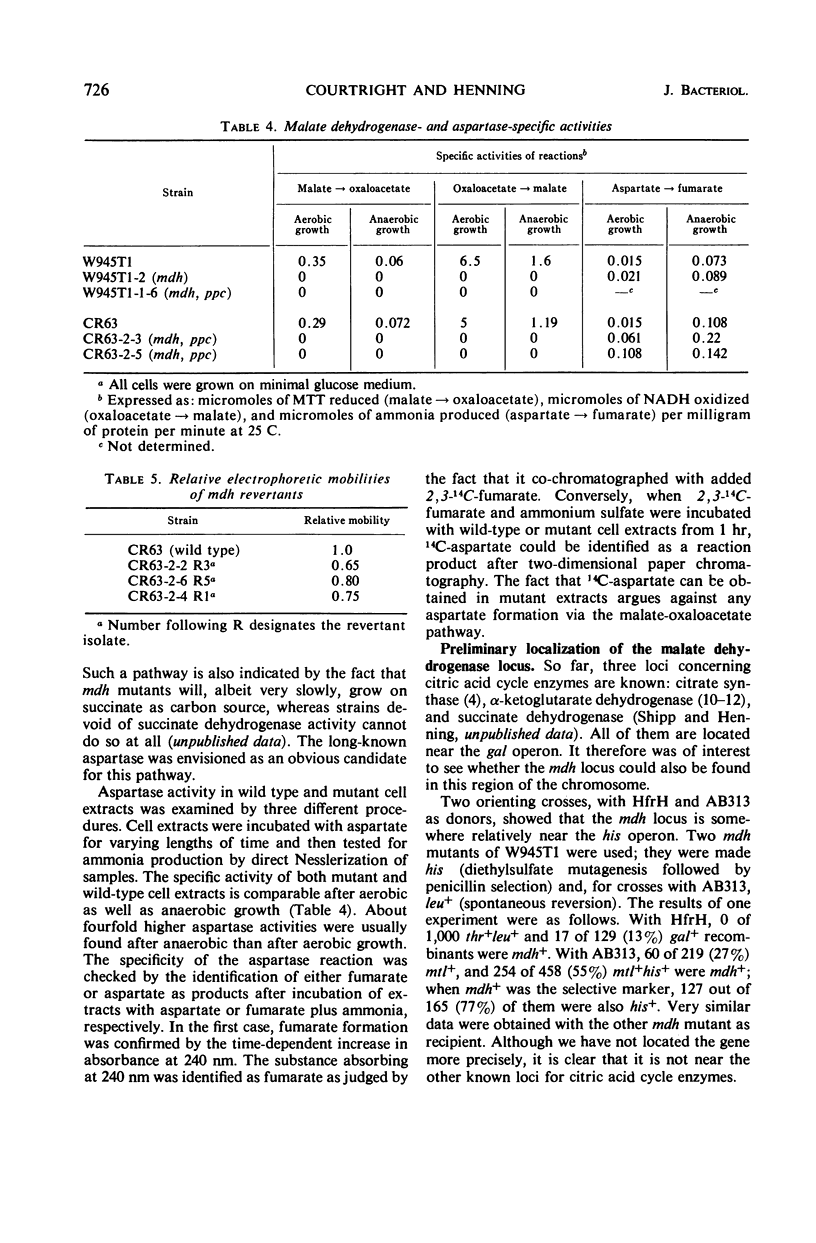
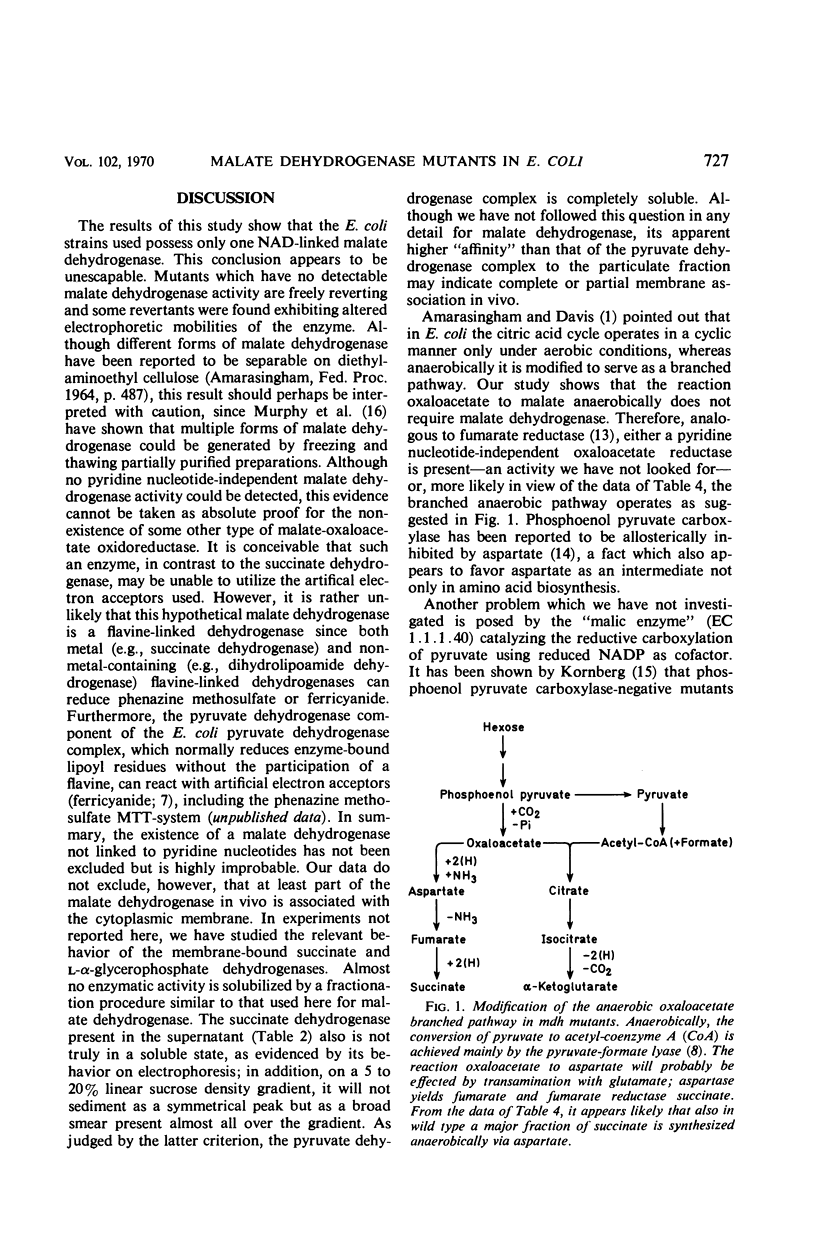
Mutants devoid of malate dehydrogenase activity have been isolated in Escherichia coli K-12. They do not possess detectable malate dehydrogenase when grown aerobically or anaerobically on glucose as sole carbon source. All mutants revert spontaneously; a few partial revertants have been found with a malate dehydrogenase exhibiting altered electrophoretic mobility. Therefore, only one such enzyme appears to exist in the strains examined. No evidence could be obtained for the presence of a malate dehydrogenase not linked to nicotinamide adenine dinucleotide. Mutants deficient in both malate dehydrogenase and phosphoenol pyruvate carboxylase activities will grow anaerobically on minimal glucose plus succinate medium; also, malate dehydrogenase mutants do not require succinate for anaerobic growth on glucose. The anaerobic pathway oxaloacetate to succinate or succinate to aspartate appears to be accomplished by aspartase. Malate dehydrogenase is coded for by a locus somewhere relatively near the histidine operon, i.e., a different chromosomal location than that known for other citric acid cycle enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- ASHWORTH J. M., KORNBERG H. L., NOTHMANN D. L. LOCATION OF THE STRUCTURAL GENE FOR CITRATE SYNTHASE ON THE CHROMOSOME OF ESCHERICHIA COLI K12. J Mol Biol. 1965 Mar;11:654–657. doi: 10.1016/s0022-2836(65)80021-3. [DOI] [PubMed] [Google Scholar]
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Snoswell A. M., Gibson F. The use of a ubiquinone-deficient mutant in the study of malate oxidation in Escherichia coli. Biochim Biophys Acta. 1968 Jan 15;153(1):1–12. doi: 10.1016/0005-2728(68)90140-0. [DOI] [PubMed] [Google Scholar]
- DAS M. L., KOIKE M., REED L. J. On the role of thiamine pyrophosphate in oxidative decarboxylation of alpha-keto acids. Proc Natl Acad Sci U S A. 1961 Jun 15;47:753–759. doi: 10.1073/pnas.47.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNING U. EIN REGULATIONSMECHANISMUS BEIM ABBAU DER BRENZTRAUBENSAEURE DURCH ESCHERICHIA COLI. Biochem Z. 1963 Jul 26;337:490–504. [PubMed] [Google Scholar]
- HENNING U., HERZ C. EIN STRUKTURGEN-KOMPLEX FUER DEN PYRUVAT-DEHYDROGENASE-KOMPLEX VON ESCHERICHIA COLI K 12. Z Vererbungsl. 1964 Nov 11;95:260–275. [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):182–190. doi: 10.1007/BF00445687. [DOI] [PubMed] [Google Scholar]
- Izui K., Iwatani A., Nishikido T., Katsuki H., Tanaka S. Regulation of phosphoenolpyruvate carboxylase activity in Escherichia coli. Biochim Biophys Acta. 1967 May 16;139(1):188–190. doi: 10.1016/0005-2744(67)90130-1. [DOI] [PubMed] [Google Scholar]
- Murphey W. H., Barnaby C., Lin F. J., Kaplan N. O. Malate dehydrogenases. II. Purification and properties of Bacillus subtilis, Bacillus stearothermophilus, and Escherichia coli malate dehydrogenases. J Biol Chem. 1967 Apr 10;242(7):1548–1559. [PubMed] [Google Scholar]
- REED L. J., LEACH F. R., KOIKE M. Studies on a lipoic acid-activating system. J Biol Chem. 1958 May;232(1):123–142. [PubMed] [Google Scholar]
- Ursprung H., Leone J. Alcohol dehydrogenases: a polymorphism in Drosophila melanogaster. J Exp Zool. 1965 Nov;160(2):147–154. doi: 10.1002/jez.1401600202. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


