Abstract
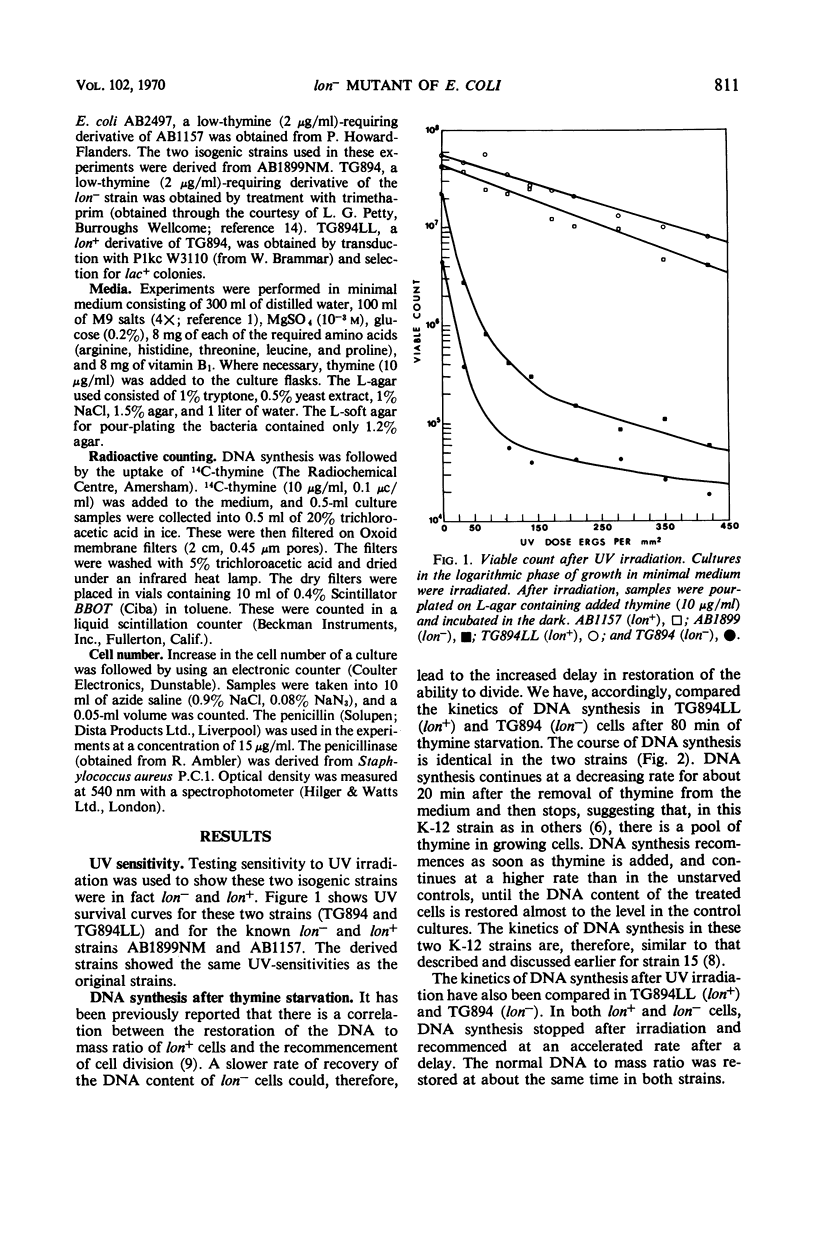
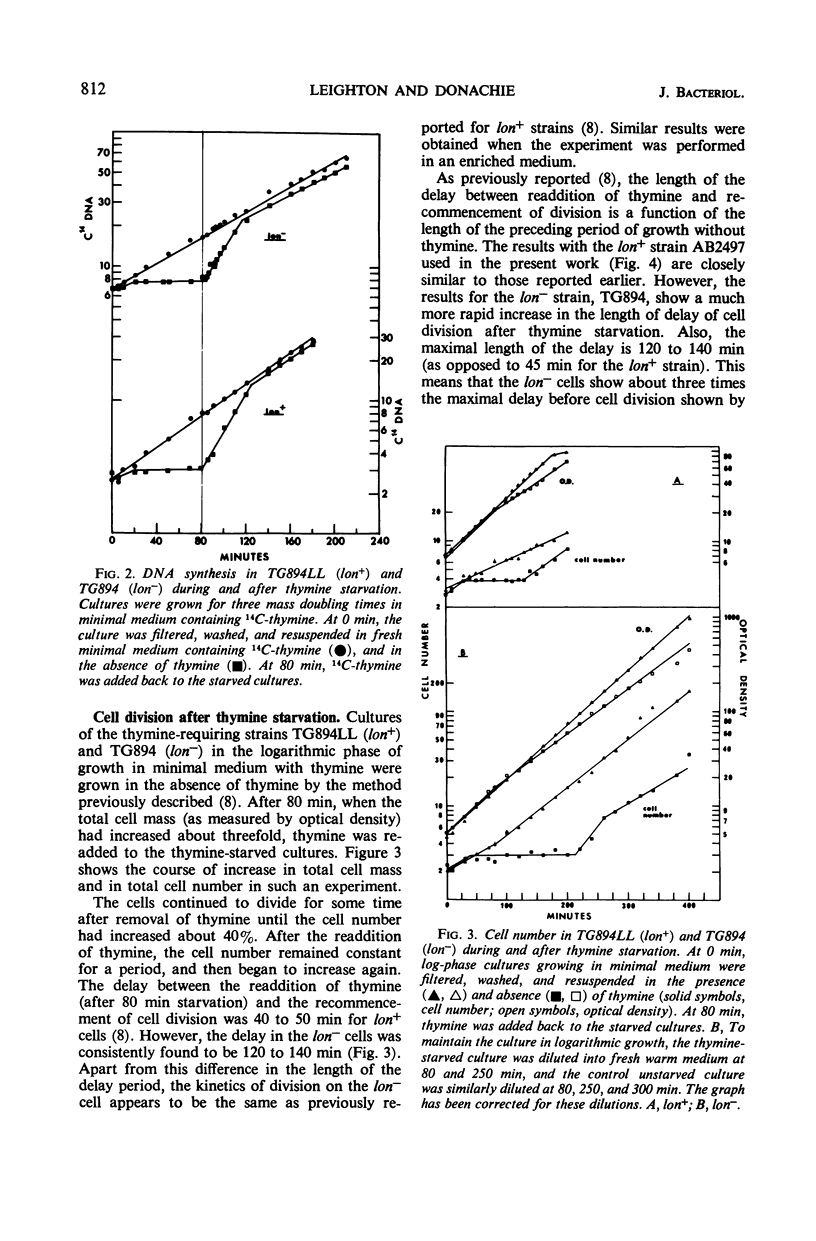
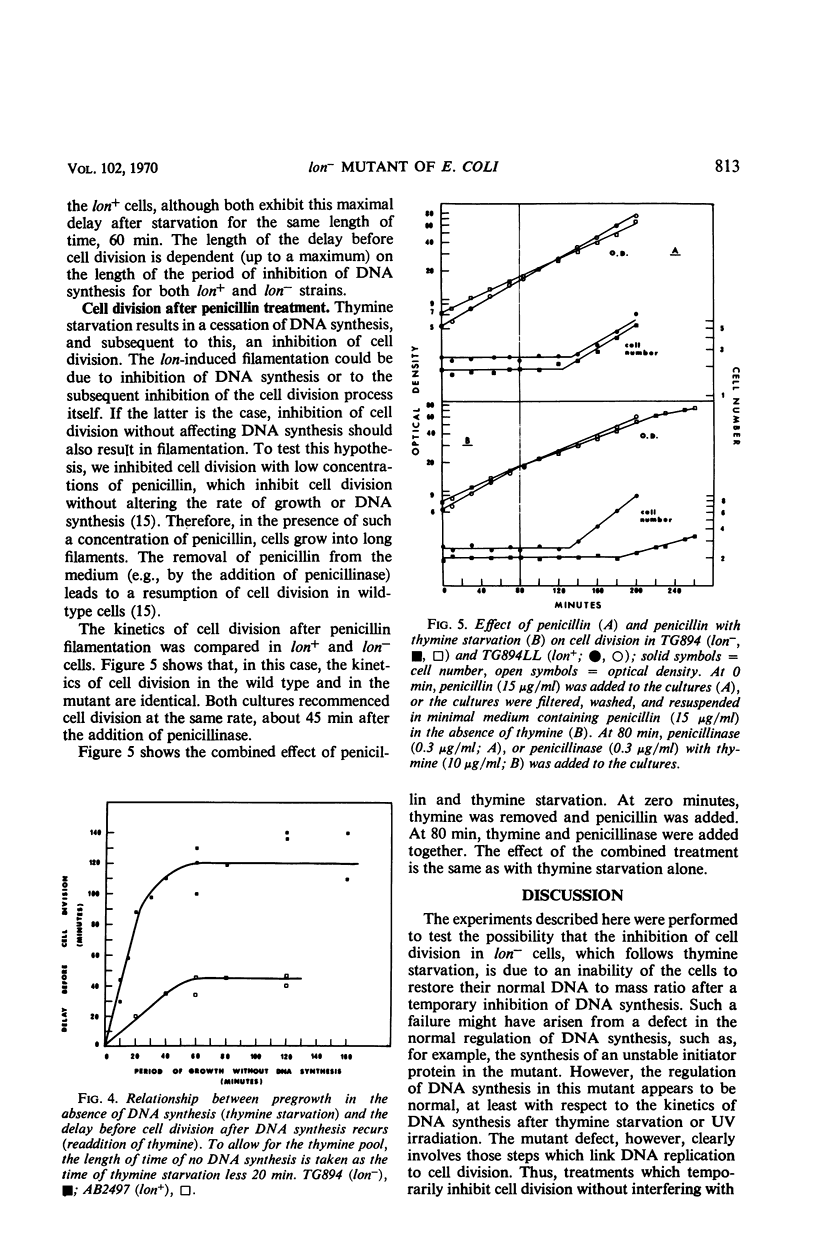
The lon− mutants of Escherichia coli form long filamentous cells after temporary inhibition of deoxyribonucleic acid (DNA) synthesis by ultraviolet irradiation, treatment with nalidixic acid, or thymine starvation. The kinetics of DNA synthesis and cell division after a period of thymine starvation have been compared in lon+ and lon− cells. After this treatment, both kinds of cells recover their normal DNA to mass ratio with the same kinetics. In contrast to previous reports, cell division is found to recommence in both lon+ and in lon− cells after such a temporary period of inhibition of DNA synthesis. However, the delay separating the recommencement of DNA synthesis and of cell division is approximately three times as long in lon− as in lon+ cells. Low concentrations of penicillin inhibit cell division in both lon+ and lon− cells. In this case, cell division recommences with the same kinetics in both strains after the removal of penicillin. This suggests that different steps in the cell division process are blocked by inhibition of DNA synthesis and by penicillin treatment. The lon− mutation appears to affect the former of these steps.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ANDERSON E. H. Heat reactivation of ultraviolet-inactivated bacteria. J Bacteriol. 1951 Apr;61(4):389–394. doi: 10.1128/jb.61.4.389-394.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Berg C. M. Chromosome replication in some strains of Escherichia coli K12. Cold Spring Harb Symp Quant Biol. 1968;33:559–573. doi: 10.1101/sqb.1968.033.01.063. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Hobbs D. G., Masters M. Chromosome replication and cell division in Escherichia coli 15T after growth in the absence of DNA synthesis. Nature. 1968 Sep 7;219(5158):1079–1080. doi: 10.1038/2191079a0. [DOI] [PubMed] [Google Scholar]
- Fisher W. D., Adler H. I., Shull F. W., Jr, Cohen A. Properties of a cell fraction that repairs damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1969 Feb;97(2):500–505. doi: 10.1128/jb.97.2.500-505.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Cellular division of penicillin-induced filaments of Escherichia coli. Folia Microbiol (Praha) 1967;12(3):240–247. doi: 10.1007/BF02868738. [DOI] [PubMed] [Google Scholar]
- VAN DE PUTTE P., WESTENBROEK C., ROERSCH A. THE RELATIONSHIP BETWEEN GENE-CONTROLLED RADIATION RESISTANCE AND FILAMENT FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 15;76:247–256. doi: 10.1016/0006-3002(63)90037-4. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


