Abstract
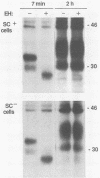
Numerous studies have indicated that a modified proteinase K-resistant form of an endogenous brain protein, prion protein (PrP), is associated with scrapie infection in animals. This scrapie-associated PrP modification appears to occur posttranslationally in brain, but its molecular nature is not known. To learn about the normal PrP biosynthesis and whether it is altered by scrapie infection in vitro, we did metabolic labeling experiments with uninfected and scrapie-infected mouse neuroblastoma tissue culture cells. Pulse-chase labeling experiments indicated that, in both cell types, two major PrP precursors of 28 and 33 kilodaltons (kDa) were processed to mature 30- and 35- to 41-kDa forms. Endoglycosidase H, tunicamycin, and phospholipase treatments revealed that the 28- and 33-kDa precursors resulted from the addition of high-mannose glycans to a 25-kDa polypeptide containing a phosphatidylinositol moiety and that maturation of the precursors involved the conversion of the high-mannose glycans to hybrid or complex glycans. Treatments of the live cells with trypsin and phosphatidylinositol-specific phospholipase C indicated that the mature PrP species were expressed solely on the cell surface, where they were anchored by covalent linkage to phosphatidylinositol. Once on the cell surface, the major PrP forms had half-lives of 3 to 6 h. No differences in PrP biosynthesis were observed between the scrapie-infected versus uninfected neuroblastoma cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T., Cramp W. A., Haig D. A., Clarke M. C. Does the agent of scrapie replicate without nucleic acid? Nature. 1967 May 20;214(5090):764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Cleaver J. E., Diener T. O., Prusiner S. B. Purified scrapie prions resist inactivation by UV irradiation. J Virol. 1987 Jan;61(1):159–166. doi: 10.1128/jvi.61.1.159-166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. Isolation and structural studies of the intact scrapie agent protein. Arch Biochem Biophys. 1987 Nov 1;258(2):579–590. doi: 10.1016/0003-9861(87)90380-8. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Meyer R. K., Prusiner S. B. Scrapie PrP 27-30 is a sialoglycoprotein. J Virol. 1985 Feb;53(2):596–606. doi: 10.1128/jvi.53.2.596-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig H. R., Diringer H. Scrapie: concept of a virus-induced amyloidosis of the brain. EMBO J. 1985 Sep;4(9):2309–2312. doi: 10.1002/j.1460-2075.1985.tb03931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Race R. E., Chesebro B. Detection of prion protein mRNA in normal and scrapie-infected tissues and cell lines. J Gen Virol. 1988 Mar;69(Pt 3):711–716. doi: 10.1099/0022-1317-69-3-711. [DOI] [PubMed] [Google Scholar]
- Caughey B., Race R. E., Vogel M., Buchmeier M. J., Chesebro B. In vitro expression in eukaryotic cells of a prion protein gene cloned from scrapie-infected mouse brain. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4657–4661. doi: 10.1073/pnas.85.13.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Antibody to scrapie-associated fibril protein identifies a cellular antigen. J Gen Virol. 1986 Feb;67(Pt 2):243–253. doi: 10.1099/0022-1317-67-2-243. [DOI] [PubMed] [Google Scholar]
- Dees C., Wade W. F., German T. L., Marsh R. F. Inactivation of the scrapie agent by ultraviolet irradiation in the presence of chlorpromazine. J Gen Virol. 1985 Apr;66(Pt 4):845–849. doi: 10.1099/0022-1317-66-4-845. [DOI] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Griffith J. S. Self-replication and scrapie. Nature. 1967 Sep 2;215(5105):1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Hay B., Barry R. A., Lieberburg I., Prusiner S. B., Lingappa V. R. Biogenesis and transmembrane orientation of the cellular isoform of the scrapie prion protein [published errratum appears in Mol Cell Biol 1987 May;7(5):2035]. Mol Cell Biol. 1987 Feb;7(2):914–920. doi: 10.1128/mcb.7.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B., Prusiner S. B., Lingappa V. R. Evidence for a secretory form of the cellular prion protein. Biochemistry. 1987 Dec 15;26(25):8110–8115. doi: 10.1021/bi00399a014. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L. J., Farquhar C. F., Multhaup G., Beyreuther K., Kimberlin R. H. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 1986 Oct;5(10):2591–2597. doi: 10.1002/j.1460-2075.1986.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Locht C., Chesebro B., Race R., Keith J. M. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6372–6376. doi: 10.1073/pnas.83.17.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. F., Malone T. G., Semancik J. S., Lancaster W. D., Hanson R. P. Evidence for an essential DNA component in the Scrapie agent. Nature. 1978 Sep 14;275(5676):146–147. doi: 10.1038/275146a0. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pattison I. H., Jones K. M. The possible nature of the transmissible agent of scrapie. Vet Rec. 1967 Jan 7;80(1):2–9. doi: 10.1136/vr.80.1.2. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Race R. E., Caughey B., Graham K., Ernst D., Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988 Aug;62(8):2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Rubenstein R., Kascsak R. J., Merz P. A., Papini M. C., Carp R. I., Robakis N. K., Wisniewski H. M. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J Gen Virol. 1986 Apr;67(Pt 4):671–681. doi: 10.1099/0022-1317-67-4-671. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Thotakura N. R., Bahl O. P. Enzymatic deglycosylation of glycoproteins. Methods Enzymol. 1987;138:350–359. doi: 10.1016/0076-6879(87)38030-9. [DOI] [PubMed] [Google Scholar]