Abstract
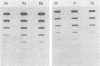
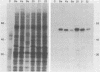
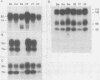
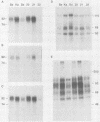
Pseudorabies virus (PRV) vaccine strain Bartha has a diminished capacity to cause disease and harbors a variety of mutations affecting virulence. It has been reported that PRV Bartha produces virions with reduced amounts of the major envelope glycoprotein gIII. One hypothesis was that this phenotype was due to reduced expression of the gIII gene. In this report, we demonstrate that the reduced amount of gIII in virions was not mediated at the level of transcription, but rather reflected a defect in protein localization. We describe experiments with gene replacement technology to prove that the expression defect was closely linked to the gIII gene itself. Using pulse-chase experiments, we found a defect similar to that observed for certain signal sequence mutations of PRV Becker gIII. The Bartha gIII protein was translated, but was inefficiently introduced into the membrane protein export pathway. Consequently, only a fraction of the primary Bartha gIII translation product was glycosylated and matured. The remaining fraction stayed presumably in the cytoplasm, where it never became glycosylated or inserted into cell or virus membranes. The result was that Bartha-infected cells produced virions with reduced amounts of gIII in their envelopes. Comparison of the DNA sequence of the promoter and amino-terminal coding regions of Becker and Bartha gIII genes revealed a single base pair difference in Bartha, changing codon 14 of the signal sequence from a leucine (CTC) to a proline (CCC) codon. We suggest that the signal sequence mutation is responsible for the apparent reduced expression phenotype of this attenuated strain. This mutation represents, to our knowledge, the first reported natural signal sequence mutation in a herpesvirus glycoprotein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., DeMarchi J. M., Lomniczi B., Kaplan A. S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986 Oct 30;154(2):325–334. doi: 10.1016/0042-6822(86)90458-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., DeMarchi J., Pendrys J., Veach R. A., Kaplan A. S. Proteins specified by the short unique region of the genome of pseudorabies virus play a role in the release of virions from certain cells. J Virol. 1986 Jan;57(1):191–196. doi: 10.1128/jvi.57.1.191-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A., van den Ouweland A., Quint W., van Oirschot J., Gielkens A. Presence of markers for virulence in the unique short region or repeat region or both of pseudorabies hybrid viruses. J Virol. 1985 Jan;53(1):89–93. doi: 10.1128/jvi.53.1.89-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Keeler C. L., Jr, Robbins A. K., Ryan J. P., Whealy M. E. An amino-terminal deletion mutation of pseudorabies virus glycoprotein gIII affects protein localization and RNA accumulation. J Virol. 1988 Oct;62(10):3565–3573. doi: 10.1128/jvi.62.10.3565-3573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Van Oirschot J. T., Berns A. J. Genome differences among field isolates and vaccine strains of pseudorabies virus. J Gen Virol. 1985 Jan;66(Pt 1):69–82. doi: 10.1099/0022-1317-66-1-69. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987 Mar;61(3):796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Rziha H. J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985 Oct;56(1):307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987 Sep;61(9):2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Kaplan A. S., Ben-Porat T., Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987 Dec;61(12):4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Gierman T. M., Post L. E. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J Virol. 1986 Dec;60(3):1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Weis J. H., Enquist L. W., Watson R. J. Construction of E. coli expression plasmid libraries: localization of a pseudorabies virus glycoprotein gene. J Mol Appl Genet. 1984;2(5):485–496. [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. P., Whealy M. E., Robbins A. K., Enquist L. W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987 Oct;61(10):2962–2972. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L. Some characteristics of four attenuated vaccine virus strains and a virulent strain of Aujeszky's disease virus. Vet Q. 1984 Sep;6(4):225–229. doi: 10.1080/01652176.1984.9693940. [DOI] [PubMed] [Google Scholar]