Abstract
Yeast telomere DNA consists of a continuous, ≈330-bp tract of the heterogeneous repeat TG1-3 with irregularly spaced, high affinity sites for the protein Rap1p. Yeast monitor, or count, the number of telomeric Rap1p C termini in a negative feedback mechanism to modulate the length of the terminal TG1-3 repeats, and synthetic telomeres that tether Rap1p molecules adjacent to the TG1-3 tract cause wild-type cells to maintain a shorter TG1-3 tract. To identify trans-acting proteins required to count Rap1p molecules, these same synthetic telomeres were placed in two short telomere mutants: yku70Δ (which lack the yeast Ku70 protein) and tel1Δ (which lack the yeast ortholog of ATM). Although both mutants maintain telomeres with ≈100 bp of TG1-3, only yku70Δ cells maintained shorter TG1-3 repeats in response to internal Rap1p molecules. This distinct response to internal Rap1p molecules was not caused by a variation in Rap1p site density in the TG1-3 repeats as sequencing of tel1Δ and yku70Δ telomeres showed that both strains have only five to six Rap1p sites per 100-bp telomere. In addition, the tel1Δ short telomere phenotype was epistatic to the unregulated telomere length caused by deletion of the Rap1p C-terminal domain. Thus, the length of the TG1-3 repeats in tel1Δ cells was independent of the number of the Rap1p C termini at the telomere. These data indicate that tel1Δ cells use an alternative mechanism to regulate telomere length that is distinct from monitoring the number of telomere binding proteins.
Telomeres are the nucleoprotein complexes that protect and allow the complete replication of chromosome ends. In most eukaryotes, telomeres consist of many copies of a short repeated sequence in which the TG-rich strand forms the 3′ end of the chromosome. In the yeast Saccharomyces cerevisiae, this sequence is TG2-3(TG)1-6 or TG1-3 whereas in humans it is TTAGGG. The number of these repeats, or telomere length, can be increased by lengthening mechanisms, such as nonreciprocal recombination or de novo synthesis by the ribonucleoprotein enzyme telomerase, and decreased by shortening mechanisms, such as incomplete replication or nucleolytic degradation of these repeats (1, 2, 3). The length of these repeats is regulated, most likely by balancing lengthening and shortening mechanisms. In yeast, the average length of the TG1-3 repeats is ≈330 bp. In humans, the TTAGGG repeat length is relatively constant at 10–20 kb in germ cells but gradually shortens in somatic cells as they undergo repeated mitotic divisions because somatic cells express little or no telomerase activity. TTAGGG repeat length acts as a “mitotic clock” that causes senescence when telomeres reach a certain length (4). How telomere length is measured is essential to understand how it serves as a metric for the number of cell divisions.
Yeast telomere length is regulated by the yeast protein Rap1p, which binds to a consensus site within the double-stranded TG1-3 repeats. Tethering arrays of Rap1p molecules or Rap1p C termini just internal to the TG1-3 repeats causes cells to maintain the TG1-3 tract at a shorter length (5, 6). These experiments indicate that cells monitor telomere length by counting the number of telomere binding proteins and that the arrays of internal Rap1p molecules or Rap1p C termini are counted as part of the TG1-3 tract. The Rap1p C-terminal domain plays an essential role in this process because deletion of this domain in rap1-17 cells causes the TG1-3 repeats to expand to 2–4 kb (7). Several proteins interact with the Rap1p C terminus, and elimination of two of these, Rif1p and Rif2p, phenocopies the unregulated telomere length of the rap1-17 mutant (8). This extreme telomere elongation is thought to result from telomerase having more frequent access to the chromosome end and synthesizing an excess of telomere repeats (9).
Yeast mutations that cause cells to maintain their TG1-3 repeats at shorter lengths provide an additional method to investigate how telomere length is regulated. Two of these mutations, tel1Δ and yku70Δ (formerly called hdf1Δ), cause cells to maintain their chromosomal TG1-3 repeats at ≈100 bp by different genetic pathways (10, 11). TEL1 encodes a protein with sequence similarity to human ATM and DNA-PK (12, 13). YKU70 encodes Yku70p, which forms a heterodimer with Yku80p, the yeast version of Ku, that binds to double-stranded DNA ends in vitro (14) and telomeres in vivo (15). The mechanism as to how each mutation alters telomere length is unclear.
Short telomere mutations could alter telomere length in two general ways. One would be to alter the process of counting telomere binding proteins: such mutants might not count telomere-bound Rap1p molecules and might regulate length of the TG1-3 repeats through an alternative mechanism. A second way would be to alter the synthesis and degradation of TG1-3 repeats: such mutants should count telomere-bound Rap1p molecules but might maintain shorter TG1-3 tracts because telomere lengthening (synthesis) is counteracted by increased telomere shortening (degradation). Here we show that the tel1Δ mutation has the properties of the first class whereas yku70Δ has the properties of the second. Length of the TG1-3 tract in tel1Δ cells was unaffected by the presence of six Rap1p molecules adjacent to the TG1-3 repeats whereas these Rap1p molecules cause yku70Δ cells to maintain a shorter TG1-3 tract. Thus, tel1Δ cells apparently use a mechanism that is independent of the number of telomere-bound Rap1p molecules to maintain a constant telomere length.
Materials and Methods
Yeast Methods.
The complete deletion of TEL1 and YKU70 ORFs from yeast strain KR36-6L (16) were constructed by using the methods of Baudin et al. (17) and Morrow et al. (13). The tel1Δ rap1-17 double mutants were made by crossing AR2-5B (MATα rap1-17 ura3-1 his3-11, 15 leu2-3, 112 trp1 ade2-1 can1-100) to KR36-6L tel1Δ∷HIS3 to generate the diploid and haploid segregants analyzed in Fig. 3. The rap1-17 allele was followed by monitoring growth at 20°C (7). Similarly, KR36-6L tel1Δ∷HIS3 was crossed with 708 tlc1Δ∷HIS3 (16) to generate the tel1Δ tlc1Δ cells. The tel1Δ∷HIS3 mutation was confirmed in all of the rap1-17 tel1Δ and tlc1Δ tel1Δ double mutants by PCR. Growth of tlc1Δ and tel1Δ tlc1Δ strains after scoring the tetrad segregants (≈30 generations of growth) was performed by streaking yeast cells for single colonies (≈20 generations of growth per streak), picking a new single colony and streaking again. PCR, telomere formation, Southern blotting, and telomere length measurement methods were previously described (6, 16).
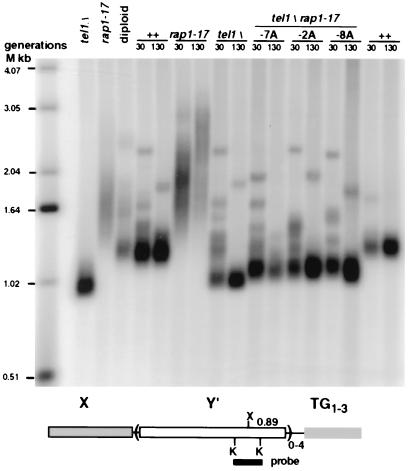
Figure 3.
The tel1Δ rap1-17 double mutant has short telomeres. Shown is a Southern blot of genomic DNA cut with XhoI and probed with a Y′ KpnI probe that detects the terminal restriction fragment of Y′ telomeres (diagrammed at the bottom of the blot) and internal Y′ tandem arrays (36). The leftmost lanes labeled tel1Δ and rap1-17 are the haploid strains used to form the diploid parent (labeled diploid). The remaining lanes show genomic DNA from haploids isolated by sporulating this diploid. The haploid progeny were grown for either 30 or 130 generations after tetrad dissection to allow for the gradual telomere shortening that occurs in tel1Δ cells. For simplicity, the genotypes of the individual TEL1 RAP1 (++) and single mutant haploids are shown (the two ++ lanes are from different strains), and the individual tel1Δ rap1-17 double mutants are indicated by their tetrad designations.
Cloning and Sequencing Telomeres.
The pYAC4 circular plasmid was digested with BamHI to expose the Tetrahymena C4A2 sequences. The 5.9-kb gel-purified fragment was transformed into KR36-6L (wild-type), KR36-6L tel1Δ∷HIS3, and KR36-6L yku70Δ∷HIS3 strains. YAC formation was confirmed by Southern blotting, and total genomic DNA was isolated from yeast cells by using a Qiagen genomic tip. About 20 μg of DNA was digested with SmaI, was treated with T4 DNA polymerase to blunt the ends, was ligated, and was transformed into yeast selecting for tryptophan prototrophy. This approach has previously allowed the recovery of long telomere inserts (18). The circularized YAC was then transformed into DH10B cells. Telomeric inserts were sequenced by the dye termination method using a 55°C annealing temperature and the primer GTT GGT TTA AGG CGC AAG AC in the Lerner Research Institute DNA Sequencing Core. The GenBank accession numbers for these sequences are AF163941–AF163970, inclusive.
Analysis of Rap1p Binding Sites.
Telomere sequences were searched for 13 of 13 bp matches to the Rap1p binding site consensus sequence ACACCCACACACC, 12 of 13 bp matches (ACACCCACACAC) distinct from the 13/13 matches, and matches to the altered site ACACCCACACCAC, which can be bound by Rap1p with high affinity (19). Standard deviations of each data set were determined by using a jackknife protocol (20) in which one telomere sequence was removed from the group of n telomere sequences to generate n sets of telomere sequences with n − 1 sequences per set. The frequency of bp of TG1-3 per Rap1p binding site was determined for each new set of sequences, and the standard deviation of these frequencies is reported.
Results
Internal Rap1p Molecules Cause Shortening of the Terminal TG1-3 Repeats in Wild-Type and yku70Δ Cells but Not in tel1Δ Cells.
We previously constructed a series of synthetic telomeres that contain six regularly spaced Rap1p binding sites adjacent to the TG1-3 tract (6). These synthetic telomeres replace the left telomere of chromosome VII (VIIL) and form a new functional telomere (Fig. 1A). The TG1-3 tract length of the new telomere depends on how the cell counts the six Rap1p sites. In wild-type cells, the length of the terminal restriction fragments of the TEF18-6TG telomere and a control telomere containing only TG1-3 sequences were indistinguishable, and the length of the TG1-3 tract was shorter on TEF18-6 telomeres (6).
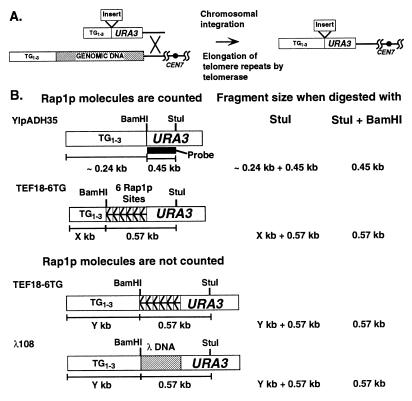
Figure 1.
Synthetic telomeres used to determine whether internal Rap1p molecules cause shortening of the terminal TG1-3 repeats in wild-type, tel1Δ, and yku70Δ cells. (A) Replacement of the left telomere of chromosome VII by homologous recombination gives rise to a functional telomere with different telomere constructs (see ref. 6). (B) Relative terminal restriction fragment lengths of the different constructs depending on whether cells count the internal Rap1p molecules as part of the telomere. The YIpADH35 telomere contains only TG1-3 sequences, the YIpADHTEF18-6TG (TEF18-6TG) telomere contains a 108-bp insert with six tandem Rap1p binding sites, and the YIpADHλ108 (λ108) telomere contains a 108-bp insert with no Rap1p binding sites (6). In vivo TG1-3 tract length was determined by subtracting the length of the StuI + BamHI fragment from the length of the StuI fragment. The portion of the URA3 gene used as the Southern blot probe is indicated by the black box. If the six internal Rap1p sites are counted as part of the TG1-3 tract, the length of the terminal StuI restriction fragments from cells bearing YIpADH35 and TEF18-6TG telomeres will be the same, and both will be shorter than the λ108 telomere. If the Rap1p sites are not counted as part of the TG1-3 tract, the TEF18-6TG telomere will be the same length as the λ108 telomere, and both will be longer than the YIpADH35 telomere.
The synthetic telomere constructions used are YIpADH35, YIpADHλ108, YIpADHTEF18-6TG (Fig. 1B), and YIpADHTEF18-6CA (not shown) (6). The YIpADH35 construct forms telomeres consisting of only TG1-3 sequences that serve as a positive control in which all of the sequences distal to the URA3 gene are counted as part of the TG1-3 tract. The YIpADHλ108 construct forms telomeres that contain both TG1-3 repeats and an insert with no Rap1p sites that is not counted by the cell as part of the TG1-3 tract. The YIpADHTEF18-6TG and CA constructs form telomeres containing terminal TG1-3 sequences and six internal Rap1p sites in the same (TG) or opposite (CA) orientation as the Rap1p sites in the TG1-3 repeats. The length of the TG1-3 tracts on these telomeres depends on whether the six internal Rap1p sites are counted by the cell as part of the telomere.
The length of the chromosomal telomere is determined by digesting genomic DNA with StuI followed by Southern blot analysis using a URA3 probe. If the TEF18-6 insert is counted by the cell as part of the TG1-3 tract, then the terminal StuI restriction fragment of the YIpADH35 and TEF18-6 telomeres will have the same length (Fig. 1B, Rap1p molecules are counted). If the TEF18-6 insert is not counted, then the terminal restriction fragments of the λ108 and TEF18-6 telomeres will have the same length (Fig. 1B, Rap1p molecules are not counted). The length of the TG1-3 tract on these telomeres is determined by cutting the genomic DNA with StuI + BamHI and subtracting the length of this fragment from the length of the StuI fragment. Telomere restriction fragments give a diffuse band because the length of the TG1-3 tract is different for different telomeres present in the band. Thus, the most intensely hybridizing portion of a telomere band corresponds to the most frequent TG1-3 lengths, which we refer to as the modal telomere length (6), and was used to measure the length of the terminal restriction fragments.
The YIpADH35, YIpADHTEF18-6TG, or CA and YIpADHλ108 constructs were transformed into congenic wild-type, tel1Δ, and yku70Δ cells, and telomere length was examined. Previous work had shown that tel1 spores derived from a TEL1/tel1 diploid initially have wild-type length telomeres that gradually shorten over 100 generations of growth to reach their shortest telomere length of ≈100 bp of TG1-3 (10), a phenomenon called phenotypic lag. Telomere formation had not been analyzed in tel1Δ and yku70Δ cells. Telomeres could be formed with the ≈100 bp tel1Δ and yku70Δ length TG1-3 tracts, or tel1Δ and yku70Δ telomeres may be formed at the ≈330 bp wild-type lengths and slowly shorten, showing phenotypic lag. Consequently, the telomere lengths in both tel1Δ and yku70Δ cells were determined after 25 and 105 generations of growth. In all cases, the modal telomere lengths after 25 and 105 generations of growth were nearly identical (Fig. 2). Thus, tel1Δ cells did not form wild-type length TG1-3 tracts that gradually shortened to 100 bp over many generations.
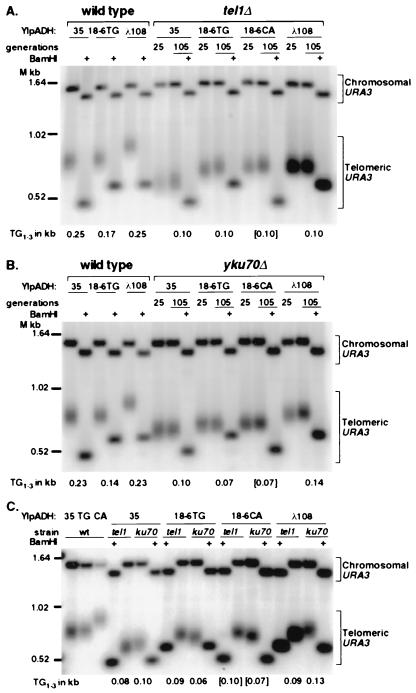
Figure 2.
tel1Δ cell telomeres do not shorten in response to additional internal Rap1p molecules whereas yku70Δ telomeres do. (A) A representative Southern blot of a 1.2% gel of YIpADH35 (labeled as 35), TEF18-6TG (18-6TG), TEF18-6CA (18-6CA), or λ108 (λ108) telomeres from wild-type and tel1Δ cells cut with StuI alone or StuI + BamHI (+). The Chromosomal URA3 band is from the ura3-52 gene on chromosome V, and the telomeric URA3 band is from the telomere constructs. In the case of tel1Δ cells, genomic DNA was prepared from cells grown for either 25 or 105 generations after transformation with the telomere construct. The length of the TG1-3 tract for the 105-generation samples, denoted at the bottom of their respective lanes, was calculated by subtracting the size of the StuI + BamHI fragment from the modal length of the StuI fragment. TEF18-6CA telomere contains the six Rap1p site insert in the opposite orientation so the BamHI site is now closer to the StuI site in URA3. This TG1-3 tract length is presented in brackets because it was calculated by using the StuI + BamHI fragment from the adjacent 18-6TG lane. (B) A representative Southern blot of wild-type and yku70Δ cells analyzed as in A. (C) The tel1Δ and yku70Δ (ku70) telomeres from cells grown for 105 generations run in adjacent lanes to show the differences in TG1-3 tract length. The TG1-3 lengths of the TEF18-6 TG and CA telomeres, measured from four blots (six tel1Δ and four yku70Δ telomeres) were 100 ± 10 bp in tel1Δ cells and 70 ± 10 bp in yku70Δ cells.
The TG1-3 tract of all tel1Δ and yku70Δ telomeres were shorter than those of wild-type cells (Fig. 2). Therefore, these synthetic telomeres were maintained at shorter lengths in response to the tel1Δ and yku70Δ mutations in the same way as natural chromosomal telomeres (10, 11).
In tel1Δ cells, the TEF18-6TG and CA telomeres gave StuI terminal restriction fragments the same length as those from λ108 telomeres and longer than the terminal restriction fragment of the YIpADH35 telomeres (Fig. 2A, tel1Δ lanes). Nine independent TEF18-6TG transformants had terminal restriction fragments of identical size by Southern blotting (data not shown). Digestion of these telomeres with StuI + BamHI showed that the structure of these telomeres in vivo was correct and that the length of the TG1-3 tract in tel1Δ cells (Fig. 2A) was 100 bp for the YIpADH35, TEF18-6TG, and λ108 telomeres. In contrast, the YIpADH35 and TEF18-6TG telomeres from wild-type cells gave StuI fragments of the same length that were shorter than the λ108 telomeric fragments (Fig. 2A, wild type). Thus, the length of the TG1-3 repeats at tel1Δ cell telomeres was not affected by increasing the number of internal Rap1p molecules.
In contrast to the tel1Δ results, the internal Rap1p sites in TEF18-6TG and CA telomeres did cause shortening of the TG1-3 tract in yku70Δ cells (Fig. 2B). The StuI fragments of these telomeres were intermediate in length between the YIpADH35 and λ108 telomeres (Fig. 2B). The lengths of the TEF18-6 TG1-3 tracts in yku70Δ cells (Fig. 2B) were 70 bp, 30 bp shorter than the yku70Δ YIpADH35 telomeres measured on the same blot. In addition, the yku70Δ TEF18-6 TG1-3 tracts were 30 bp shorter than the tel1Δ TEF18-6 TG1-3 tracts (Fig. 2C), demonstrating that the six internal Rap1p sites caused the yku70Δ cells, but not tel1Δ cells, to maintain their TG1-3 repeats at shorter lengths. Interestingly, the length of the λ108 TG1-3 tract was longer than the YIpADH35 TG1-3 tract. This length difference was seen in several independent yku70Δ λ108 telomeres, but not in any wild-type or tel1Δ λ108 telomeres (Fig. 2; data not shown; ref. 6), suggesting that this unusual λ108 tract length was specific to yku70Δ cells.
Telomeres from tel1Δ and yku70Δ Cells Have a Similar Density of Rap1p Sites Compared with Wild-Type Cells.
An alternative explanation for the failure of tel1Δ cell telomeres to shorten upon the introduction of internal Rap1p molecules would be that the telomere synthesis in tel1Δ cells is altered so that the density of Rap1p sites in the TG1-3 repeats is extremely high. In this case, the ≈100-bp tel1Δ cell telomeres would contain as many or more Rap1p sites than the ≈250-bp wild-type cell telomeres (Fig. 2A), so the number of Rap1p molecules in wild-type and tel1Δ telomeres would be the same. In contrast, because the yku70Δ cell telomeres did shorten upon the introduction of internal Rap1p sites, telomere sequences from yku70Δ cells should have a density of Rap1p sites more similar to that of wild-type cells.
To determine whether the Rap1p site densities of tel1Δ and yku70Δ telomeres were different from those of wild-type cells, newly formed telomeres from wild-type, tel1Δ, or yku70Δ cells were isolated, sequenced, and analyzed for the number of Rap1p binding sites (Table 1). The analysis was limited to the telomeres that were cloned, propagated, and sequenced in this work, and therefore subjected to the same manipulations. Three types of Rap1p binding sites in the TG1-3 repeats were searched for based on in vitro DNA protection studies and the crystal structure of the Rap1p DNA binding domain (19, 21): a 13 of 13 bp match to the Rap1p consensus DNA binding site, a 12 of 13 bp match, and a rearranged 13-bp site that has an 11 of 13 bp match.
Table 1.
Frequency of Rap1p binding sites in telomeres from wild-type, tellΔ, and yku70Δ cells
| Strain | Number sequenced | TG1-3, bp
|
No. of Rap1p site matches†
|
bp TG1-3/ Rap1p site‡ | ||||
|---|---|---|---|---|---|---|---|---|
| Longest* | Shortest* | Total* | 13/13 | 12/13 | 13 alt. | |||
| Wild type | 10 | 317 | 138 | 2,487 | 84 | 43 | 29 | 15.9 ± 0.2 |
| tellΔ | 22 | 227 | 20 | 1,296 | 34 | 20 | 9 | 20.6 ± 0.5 |
| yku70Δ | 11 | 272 | 67 | 1,347 | 38 | 24 | 17 | 17.1 ± 0.2 |
The longest and shortest TG1-3 tracts and total number of base pairs sequenced.
The number of matches to ACACCCACACACC, ACACCCACACAC not included in the 13/13 matches and ACACCCACACCAC, respectively.
The total TG13 sequenced divided by the sum of the 13/13, 12/13, and 13 alt. Rap1p sites ± the standard deviations.
The frequency of high affinity Rap1p DNA binding sites in either tel1Δ cell or yku70Δ cell telomeres was not increased compared with wild-type cell telomeres (Table 1). These data suggest that the ≈250 bp YIpADH35 telomeres in wild-type cells should contain ≈15 Rap1p molecules whereas the ≈100-bp telomeres of tel1Δ and yku70Δ cells should contain ≈5 and 6 Rap1p molecules, respectively.
Maintenance of Constant TG1-3 Length in tel1Δ Cells Does Not Require the Rap1p C Terminus.
The Rap1p C-terminal domain can stimulate elongation of short telomeres (16), is counted to regulate telomere length (5), and acts to prevent telomere elongation (7, 22). The rap1-17 mutation deletes the C-terminal 165 amino acids of Rap1p and causes the TG1-3 tract to increase from ≈330 bp to 2–4 kb (7). If the tel1Δ mutation causes telomere shortening by eliminating the cell's ability to count Rap1p C termini, thereby making the status of the Rap1p C terminus irrelevant to telomere length control, then a tel1Δ rap1-17 double mutant will have tel1Δ length telomeres. However, if tel1Δ cells have short telomeres because the proteins that bind to the Rap1p C terminus are altered, then the tel1Δ rap1-17 double mutant will have rap1-17 length telomeres because the telomere-bound Rap1p molecules in both rap1-17 and tel1Δ rap1-17 cells have the same structure.
Several tel1Δ rap1-17 double mutants were constructed and analyzed. To allow for the tel1Δ phenotypic lag for telomere shortening, we analyzed genomic DNA from strains grown for 30 and 130 generations. The terminal restriction fragments of the three tel1Δ rap1-17 double mutants analyzed were shorter than both the rap1-17 and wild-type terminal fragments and were close to the size of tel1Δ terminal fragments (Fig. 3). Thus, the tel1Δ mutation blocked the unregulated telomere length phenotype caused by the rap1-17 mutation. A similar conclusion has been reached independently by others (23). These data, combined with the constant TG1-3 tract length on tel1Δ synthetic telomeres (Fig. 2A), indicate that the number of Rap1p molecules and the number of Rap1p C termini at telomeres were not the determinant of telomere length in tel1Δ cells. Thus, although wild-type cells maintain a constant number of the Rap1p molecules to regulate telomere length, tel1Δ cells maintain a constant number of TG1-3 sequences by an alternative, unknown mechanism.
Telomere Maintenance in tel1Δ Cells Requires Telomerase RNA.
Telomere elongation in yeast occurs predominantly via telomerase because elimination of telomerase activity leads to the gradual shortening of telomeres until cells senesce after ≈60 generations (24, 25). Cells deficient in yku70 and telomerase activity undergo senescence in 20–40 generations, indicating a strong requirement for telomerase in yku70 cells (15, 26). Telomerase most likely elongates telomeres in tel1Δ cells. We have shown that a short 29-bp TG1-3 tract forms telomeres at low efficiency in yeast cells whereas this 29-bp TG1-3 tract plus six internal Rap1p binding sites forms telomeres at high efficiency in a telomerase-dependent manner (16). Cells with the tel1Δ genotype behaved the same as wild-type cells in the telomere formation assay, indicating that internal Rap1p molecules can stimulate telomere formation in tel1Δ cells (ref. 16; data not shown). However, that work did not determine whether the Rap1p-stimulated telomere formation in tel1Δ cells used telomerase or a recombination-based telomere lengthening mechanism (27). If tel1Δ cells were using recombination to elongate their telomeres to a large degree, then tel1Δ cells that lack telomerase activity should survive many generations longer than wild-type cells lacking telomerase activity.
To determine the extent of the telomerase requirement of tel1Δ cells, tel1Δ cells were crossed to tlc1Δ cells (which lack the gene for telomerase RNA) to generate wild-type, tel1Δ, tlc1Δ, and tel1Δ tlc1Δ haploid progeny. All of these haploids arose from spores that were born with wild-type length telomeres generated in the phenotypically wild-type diploid. These four cell types were then grown by serial restreaking to determine when cells would begin to senesce. As expected, wild-type and tel1Δ cells grew well throughout the experiment and did not senesce whereas tlc1Δ cells began to produce colonies of senescent cells (i.e., small, irregularly shaped colonies that do not enlarge after prolonged incubation) after 50 generations of growth (ref. 24; Fig. 4; data not shown). The tel1Δ tlc1Δ double mutant cells also began to produce colonies of senescent cells after slightly more than 50 generations of growth, as evidenced by the larger senescent colonies they produced (Fig. 4). The tel1Δ tlc1Δ cells formed smaller colonies than the tel1Δ and wild-type colonies and produced a higher frequency of senescent colonies on the next restreak (data not shown). Four tlc1Δ spores and four tel1Δ tlc1Δ spores were examined, and the tel1Δ tlc1Δ cells always required ≈10 generations more growth than tlc1Δ cells to manifest the senescent phenotype. Similar results have been obtained by others (28). This extended life span could reflect an increase in recombination-mediated telomere elongation because tel1Δ cells have a 3-fold higher rate of mitotic recombination over wild-type cells (12). Thus, although tel1Δ tlc1Δ cells did survive longer than the tlc1Δ cells, the difference in the number of generations was small, and tel1Δ tlc1Δ cells clearly did senesce. These results indicate that tel1Δ cells cannot maintain their TG1-3 repeats in the absence of telomerase activity. Therefore, the alternative mechanism that tel1Δ cells use to maintain a constant telomere length without counting Rap1p molecules still requires telomerase (Fig. 4).
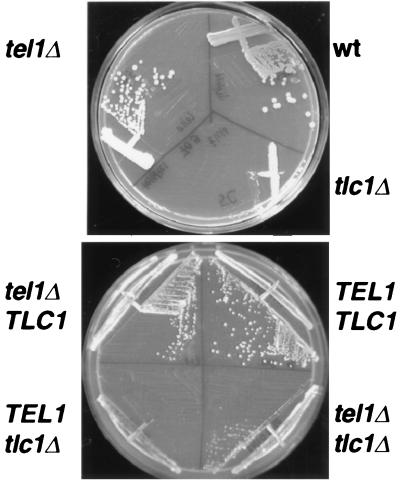
Figure 4.
Both tlc1Δ and tel1Δ tlc1Δ cells form senescent colonies after 50 generations of growth. The top plate shows KR36-6L (wt), the tel1Δ and tlc1Δ haploid parents streaked for single colonies. The tlc1Δ strain had previously grown ≈50 generations without the TLC1 gene before streaking on this plate. The bottom plate shows a representative sample of four haploid progeny derived from a TEL1/tel1Δ TLC1/tlc1Δ diploid in which all strains had been grown for ≈50 generations before streaking on this plate.
Discussion
The length of the telomeric TG1-3 tract is thought to be regulated by a negative feedback loop that maintains a constant number of telomere binding proteins because the tethering of Rap1p molecules or Rap1p C termini adjacent to the TG1-3 repeats causes cells to maintain these repeats at a shorter length (5, 6). To begin to identify the components that control this negative feedback loop, we tested two pathways previously identified by mutations that cause short telomeres (10, 11). The TG1-3 tracts of yku70Δ cells were maintained at shorter lengths in response to six internal Rap1p molecules, indicating that the system for counting Rap1p molecules to measure telomere length was intact in these cells. The alteration of TG1-3 length was different in yku70Δ cells and wild-type cells (Fig. 2B), suggesting that either the counting system was altered in yku70Δ cells or that the counting system is normal but that these cells elongate their TG1-3 repeats further to fulfill a telomere function distinct from length regulation [e.g., efficient replication, CA-strand degradation (2), or Cdc13p or Tel2p binding (29, 30)]. These two possibilities cannot currently be distinguished. In contrast to yku70Δ and wild-type cells, the TG1-3 tracts of tel1Δ cells were not altered by internal Rap1p molecules (Fig. 2A). The tel1Δ mutation was largely epistatic to the uncontrolled telomere elongation caused by deletion of the Rap1p C terminus (Fig. 3). A simple explanation for these data is that the tel1Δ mutation disrupts the system for counting Rap1p C termini to measure telomere length and that Tel1p is a necessary component of the counting system.
The tel1Δ mutation could disrupt telomere length measurement by changing the rates of telomere shortening or elongation. How telomere shortening due to degradation is regulated is not known. Lengthening and shortening due to incomplete replication are thought to be altered by changing telomerase access to the chromosome end. Models for telomere length regulation posit that, when the number of telomere bound proteins reaches a certain minimum number, telomerase access to the 3′ end of the chromosome is no longer allowed, so telomere elongation is inhibited (5, 6). Recent data indicate that the rate of telomere elongation is inversely proportional to telomere length (31). We have suggested that, when telomeres reach a minimum length, they can form a DNA-protein structure that blocks elongation (6). The slower elongation rate of longer telomeres (31) could signify the ability of a subpopulation of telomeres to form this structure and block lengthening. Tel1p may regulate telomere length by affecting how this structure forms. In wild-type cells, Tel1p action would allow TG1-3 addition to telomeres containing ≈200 bp of TG1-3 but not to telomeres with >350 bp of TG1-3. In tel1Δ cells, telomerase access appears to be blocked when telomeres are only ≈100 bp in length. Thus, TEL1 would alter telomere length regulation by altering telomere chromatin structure.
An alternative explanation for the tel1Δ results is that the absence of Tel1p somehow alters the properties of the telomerase enzyme so that TG1-3 tracts longer than ≈100 bp cannot be synthesized. In this case, the altered telomerase activity in tel1Δ cells would have to affect the elongation of both long and short telomeres differently, such that telomerase polymerization activity would have to decrease with the length of the telomere, independent of the access to the chromosome end. Such an alteration in activity would be distinct from constant telomerase processivity in which the access to the end is regulated.
Our results raise the question as to how short telomere mutants maintain a constant telomere length. In yku70Δ cells, the Rap1p counting system is present, so telomere length is most probably maintained by an altered version of the system used in wild-type cells. In contrast, tel1Δ cells may well lack the Rap1p counting system, and so must use an alternative mechanism to maintain a constant telomere length. Thus, yeast have two pathways to ensure that telomeres are maintained at a constant length.
Besides tel1Δ rap1-17 cells, yeast bearing the recently described “humanized” yeast telomerase RNA allele (32) also appear to regulate telomere length in the absence of Rap1p C termini at the chromosome end. The yeast telomerase RNA template has been mutated to produce mammalian telomere repeats (the tlc1-hs allele) and has been introduced into yeast as the only functional TLC1 gene. The resulting yeast contain hybrid telomeres in which the internal sequences are TG1-3 repeats and the terminal sequences are TTAGGG (32). Surprisingly, given the fact that Rap1p cannot bind to TTAGGG sequences (33), the length of these telomeres are constant and are approximately the same length as the tel1Δ cell telomeres (32). In both tel1Δ and tlc1-hs cells, internal Rap1p molecules bound to TG1-3 repeats can still recruit telomerase (16), and the Cdc13p can bind to single-stranded TG overhangs, either TG1-3 or TTAGGG (29). These considerations suggest a telomere length regulation model in which the internal Rap1p molecules bound to TG1-3 repeats attract telomerase to the telomere. Because the Rap1p-dependent activities that regulate length are defective in tel1Δ and tlc1-hs cells, telomere elongation and shortening would be limited by Cdc13p binding to the single-stranded TG overhang made by telomerase. In wild-type cells, Cdc13p binding protects telomeres from degradation (34) and may act as a negative regulator of telomerase when bound by Stn1p to limit elongation (35). Thus, the proposed alternative length regulation mechanism would involve recruitment of telomerase by internal Rap1p molecules followed by limitation of telomerase elongation and protection from degradation by single-stranded telomere binding proteins. The short telomere repeats seen in tel1Δ and tlc1-hs cells would result from the equilibrium between telomere elongation and degradation without the regulation derived from Rap1p and its associated proteins at the chromosome terminus.
Acknowledgments
We thank Kathleen L. Berkner for important suggestions throughout the course of this work, Nilanjan Roy for the yku70Δ strain, and Richard Gronostajski and Alex Almasan for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM50752 to K.W.R. A.R. is supported by a Leukemia Society of America Special Fellowship.
Footnotes
References
- 1.Zakian V A. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- 2.Zakian V A. Annu Rev Genet. 1996;30:141–172. doi: 10.1146/annurev.genet.30.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Greider C W. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 4.Greider C W. Curr Biol. 1998;8:R178–R181. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 5.Marcand S, Gilson E, Shore D. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 6.Ray A, Runge K W. Mol Cell Biol. 1999;19:31–45. doi: 10.1128/mcb.19.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrion G, Boakye K A, Lustig A J. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wotton D, Shore D. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 9.Krauskopf A, Blackburn E H. Proc Natl Acad Sci USA. 1998;95:12486–12491. doi: 10.1073/pnas.95.21.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustig A J, Petes T D. Proc Natl Acad Sci USA. 1986;83:1398–1402. doi: 10.1073/pnas.83.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter S E, Greenwell P W, Ritchie K B, Petes T D. Nucleic Acids Res. 1996;24:582–585. doi: 10.1093/nar/24.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwell P A, Kronmal S L, Porter S E, Gassenhuber J, Obermaier B, Petes T D. Cell. 1995;82:823–829. doi: 10.1016/0092-8674(95)90479-4. [DOI] [PubMed] [Google Scholar]
- 13.Morrow D M, Tagle D A, Shiloh Y, Collins F S, Hieter P. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 14.Feldmann H, Winnacker E L. J Biol Chem. 1993;268:12895–12900. [PubMed] [Google Scholar]
- 15.Gravel S, Larrivee M, Labrecque P, Wellinger R J. Science. 1998;280:741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 16.Ray A, Runge K W. Mol Cell Biol. 1998;18:1284–1295. doi: 10.1128/mcb.18.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baudin A, Ozier K O, Denouel A, Lacroute F, Cullin C. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runge K W, Zakian V A. Mol Cell Biol. 1989;9:1488–1497. doi: 10.1128/mcb.9.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser S M. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- 20.Weir B S. Genetic Data Analysis: Methods for Discrete Population Genetic Data. Sunderland, MA: Sinauer; 1990. [DOI] [PubMed] [Google Scholar]
- 21.Konig P, Giraldo R, Chapman L, Rhodes D. Cell. 1996;85:125–136. doi: 10.1016/s0092-8674(00)81088-0. [DOI] [PubMed] [Google Scholar]
- 22.Conrad M N, Wright J H, Wolf A J, Zakian V A. Cell. 1990;63:739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 23.Craven R J, Petes T D. Genetics. 1999;152:1531–1541. doi: 10.1093/genetics/152.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 25.Lendvay T S, Morris D K, Sah J, Balasubramanian B, Lundblad V. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent C I, Bosco G, Ross L O, Evans S K, Salinger A P, Moore J K, Haber J E, Lundblad V. Curr Biol. 1998;8:657–660. doi: 10.1016/s0960-9822(98)70253-2. [DOI] [PubMed] [Google Scholar]
- 27.Pluta A F, Zakian V A. Nature (London) 1989;337:429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- 28.Ritchie K, Mallory J, Petes T D. Mol Cell Biol. 1999;19:6065–6075. doi: 10.1128/mcb.19.9.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nugent C I, Hughes T R, Lue N F, Lundblad V. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- 30.Kota R S, Runge K W. Chromosoma. 1999;108:278–290. doi: 10.1007/s004120050379. [DOI] [PubMed] [Google Scholar]
- 31.Marcand S, Brevet V, Gilson E. EMBO J. 1999;18:3509–3519. doi: 10.1093/emboj/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henning K A, Moskowitz N, Ashlock M A, Liu P P. Proc Natl Acad Sci USA. 1998;95:5667–5671. doi: 10.1073/pnas.95.10.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z P, Tye B K. Genes Dev. 1991;5:49–59. doi: 10.1101/gad.5.1.49. [DOI] [PubMed] [Google Scholar]
- 34.Garvik B, Carson M, Hartwell L. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grandin N, Reed S I, Charbonneau M. Genes Dev. 1997;11:512–527. doi: 10.1101/gad.11.4.512. [DOI] [PubMed] [Google Scholar]
- 36.Chan C S M, Tye B-K. Cell. 1983;33:563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]