Abstract
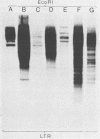
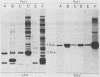
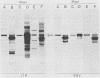
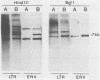
Extra proviral copies of mouse mammary tumor virus (MMTV) are known to be present in the genomes of certain T-cell lymphomas of mice. Analysis of additional non-mammary tumor cell types known to express MMTV transcripts and antigens revealed the presence of extra acquired MMTV proviruses in a pituitary tumor cell line, a macrophage line, and Leydig testicular tumor cells. The nature of the amplified MMTV proviruses in these various tumor cell types differed with regard to copy number and presence of alterations in the long terminal repeat region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J. K., Diggelmann H., Dekaban G. A., Grossi G. F., Semmler R., Waight P. A., Fletcher R. F. Alterations in the U3 region of the long terminal repeat of an infectious thymotropic type B retrovirus. J Virol. 1988 Aug;62(8):2985–2993. doi: 10.1128/jvi.62.8.2985-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Darbre P., Page M., King R. J. Androgen regulation by the long terminal repeat of mouse mammary tumor virus. Mol Cell Biol. 1986 Aug;6(8):2847–2854. doi: 10.1128/mcb.6.8.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J., Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984 Jan;49(1):92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. F., Pohajdak B., Talbot D. J., Shaw J., Paetkau V. Phorbol diester-inducible, cyclosporine-suppressible transcription from a novel promoter within the mouse mammary tumor virus env gene. J Virol. 1988 Apr;62(4):1373–1380. doi: 10.1128/jvi.62.4.1373-1380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. Mouse mammary tumor virus-related sequences in mouse lymphocytes are inducible by 12-O-tetradecanoyl phorbol-13-acetate. J Virol. 1984 Dec;52(3):1000–1004. doi: 10.1128/jvi.52.3.1000-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Prakash O., Klein D., Sarkar N. H. Structural alterations in the long terminal repeat of an acquired mouse mammary tumor virus provirus in a T-cell leukemia of DBA/2 mice. Virology. 1987 Jul;159(1):39–48. doi: 10.1016/0042-6822(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Hilkens J., Hilgers J., Groner B., Hynes N. E. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982 Sep;43(3):819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E. Site-specific rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in murine T-cell leukemias. Virology. 1986 Oct 15;154(1):76–84. doi: 10.1016/0042-6822(86)90431-9. [DOI] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Weijers P. Rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in T-cell leukemias of mouse strain GR result in a novel enhancer-like structure. Mol Cell Biol. 1985 Apr;5(4):823–830. doi: 10.1128/mcb.5.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Pourreau-Schneider N., Stephens R. J., Gardner W. U. Viral inclusions and other cytoplasmic components in a Leydig cell murine tumor: an electron microscopic study. Int J Cancer. 1968 Jan 15;3(1):155–162. doi: 10.1002/ijc.2910030119. [DOI] [PubMed] [Google Scholar]
- Racevskis J. Expression of the protein product of the mouse mammary tumor virus long terminal repeat gene in phorbol ester-treated mouse T-cell-leukemia cells. J Virol. 1986 May;58(2):441–449. doi: 10.1128/jvi.58.2.441-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Prakash O. Proteins encoded by the long terminal repeat region of mouse mammary tumor virus: identification by hybrid-selected translation. J Virol. 1984 Sep;51(3):604–610. doi: 10.1128/jvi.51.3.604-610.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Haisma H., Hilgers J. AtT20 pituitary tumour cells contain mouse mammary tumour virus and intracisternal A-type particles in addition to murine leukemia virus. Eur J Cell Biol. 1985 Nov;39(1):224–231. [PubMed] [Google Scholar]
- Wellinger R. J., Garcia M., Vessaz A., Diggelmann H. Exogenous mouse mammary tumor virus proviral DNA isolated from a kidney adenocarcinoma cell line contains alterations in the U3 region of the long terminal repeat. J Virol. 1986 Oct;60(1):1–11. doi: 10.1128/jvi.60.1.1-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasamura Y., Tashjian A. H., Jr, Sato G. H. Establishment of four functional, clonal strains of animal cells in culture. Science. 1966 Dec 2;154(3753):1186–1189. doi: 10.1126/science.154.3753.1186. [DOI] [PubMed] [Google Scholar]