Abstract
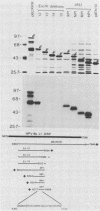
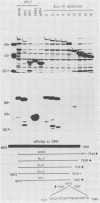
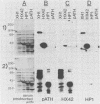
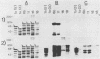
Recombinant proteins encoded by the human papillomavirus type 6b (HPV6b) L1 open reading frame react with sera from patients with condylomata acuminata and also react with rabbit antiserum raised against sodium dodecyl sulfate-disrupted bovine papillomavirus type 1 (BPV1) virions. To map the immunoreactive epitopes, a series of procaryotic expression plasmids was made which contained a nested set of 3' to 5' deletions in the HPV6b L1 open reading frame. The deleted plasmids expressed a set of carboxy to amino terminus truncated fusion proteins. Regions containing the immunoreactive epitopes were mapped by determining which of the deleted fusion proteins retained reactivity with sera in Western immunoblot assays. The coding sequence for a human antibody-reactive linear epitope mapped between HPV6b nucleotide coordinates 7045 and 7087, and the rabbit anti-BPV1-reactive epitope coding sequence mapped between coordinates 6377 and 6454. Synthetic peptides derived from the epitope mapping were reacted with sera in enzyme-linked immunosorbent assay. Human sera reacted with synthetic peptide QSQAITCQKPTPEKEKPDPYK (HPV6b L1 amino acids 417 through 437). Rabbit anti-BPV1 and rabbit antisera raised against HPV16 L1 recombinant proteins reacted with the synthetic peptide DGDMVDTGFGAMNFADLQTNKSDVPIDI (HPV6b L1 amino acids 193 through 220). Human sera which reacted with HPV6b L1 fusion proteins cross-reacted with an HPV11 L1 fusion protein but did not react with fusion proteins encoded by HPV1a, HPV16, or HPV18. Rabbit anti-BPV1 reacted with L1 fusion proteins encoded by all of these HPV types. In contrast to the type-common (rabbit anti-BPV1-reactive) epitope, the human antibody-reactive epitope appears to be relatively HPV type specific.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks L., Matlashewski G., Pim D., Churcher M., Roberts C., Crawford L. Expression of human papillomavirus type 6 and type 16 capsid proteins in bacteria and their antigenic characterization. J Gen Virol. 1987 Dec;68(Pt 12):3081–3089. doi: 10.1099/0022-1317-68-12-3081. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., zur Hausen H. Human papillomaviruses in Buschke-Löwenstein tumors: physical state of the DNA and identification of a tandem duplication in the noncoding region of a human papillomavirus 6 subtype. J Virol. 1986 Jun;58(3):963–966. doi: 10.1128/jvi.58.3.963-966.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Clad A., Gissmann L., Meier B., Freese U. K., Schwarz E. Molecular cloning and partial nucleotide sequence of human papillomavirus type 1a DNA. Virology. 1982 Apr 15;118(1):254–259. doi: 10.1016/0042-6822(82)90341-5. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987 Feb 20;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Cowsert L. M., Lake P., Jenson A. B. Topographical and conformational epitopes of bovine papillomavirus type 1 defined by monoclonal antibodies. J Natl Cancer Inst. 1987 Nov;79(5):1053–1057. [PubMed] [Google Scholar]
- Danos O., Engel L. W., Chen E. Y., Yaniv M., Howley P. M. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J Virol. 1983 May;46(2):557–566. doi: 10.1128/jvi.46.2.557-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartmann K., Schwarz E., Gissmann L., zur Hausen H. The nucleotide sequence and genome organization of human papilloma virus type 11. Virology. 1986 May;151(1):124–130. doi: 10.1016/0042-6822(86)90110-8. [DOI] [PubMed] [Google Scholar]
- De Jong A. R., Weiss J. C., Brent R. L. Condyloma acuminata in children. Am J Dis Child. 1982 Aug;136(8):704–706. doi: 10.1001/archpedi.1982.03970440048013. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firzlaff J. M., Kiviat N. B., Beckmann A. M., Jenison S. A., Galloway D. A. Detection of human papillomavirus capsid antigens in various squamous epithelial lesions using antibodies directed against the L1 and L2 open reading frames. Virology. 1988 Jun;164(2):467–477. doi: 10.1016/0042-6822(88)90561-2. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., deVilliers E. M., zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982 Feb 15;29(2):143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- Gissmann L., zur Hausen H. Partial characterization of viral DNA from human genital warts (Condylomata acuminata). Int J Cancer. 1980 May 15;25(5):605–609. doi: 10.1002/ijc.2910250509. [DOI] [PubMed] [Google Scholar]
- Grussendorf-Conen E. I., Gissmann L., Hölters J. Correlation between content of viral DNA and evidence of mature virus particles in HPV-1, HPV-4, and HPV-6 induced virus acanthomata. J Invest Dermatol. 1983 Dec;81(6):511–513. doi: 10.1111/1523-1747.ep12522845. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Wu R. New rapid methods for DNA sequencing based in exonuclease III digestion followed by repair synthesis. Nucleic Acids Res. 1982 Mar 25;10(6):2065–2084. doi: 10.1093/nar/10.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C. L., Galloway D. A. Identification of the E5 open reading frame of human papillomavirus type 16. J Virol. 1988 Mar;62(3):1071–1075. doi: 10.1128/jvi.62.3.1071-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
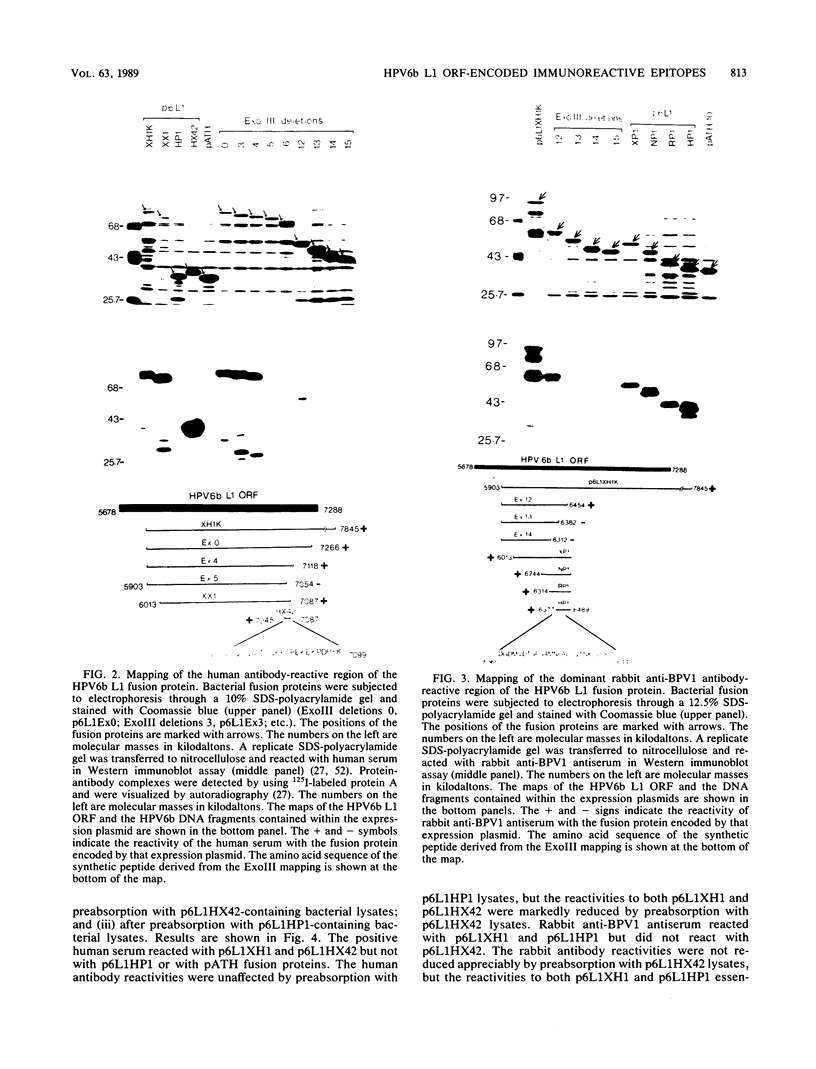
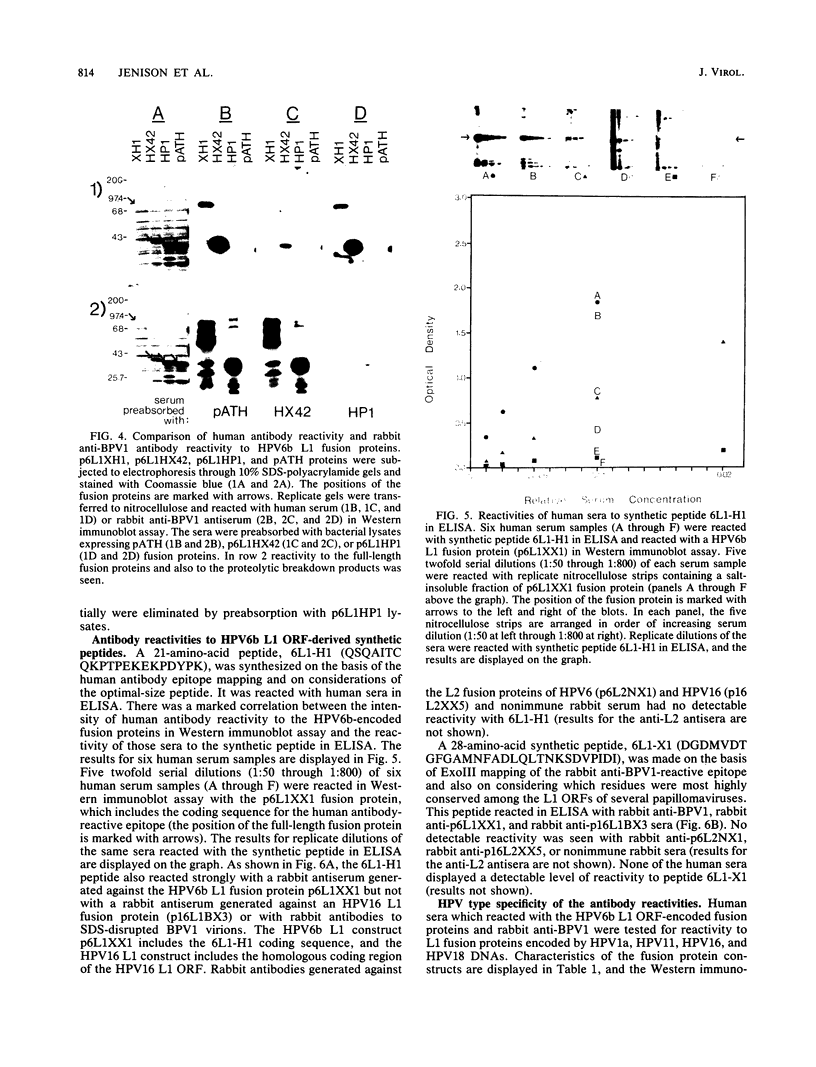
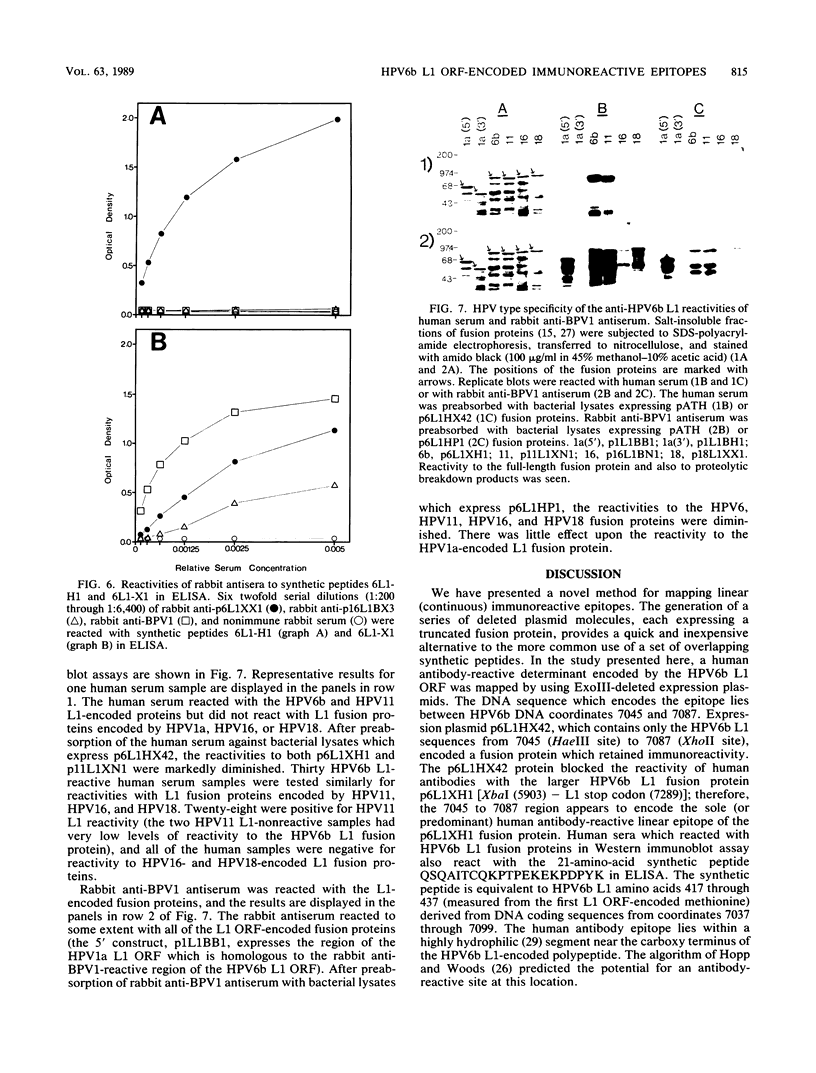
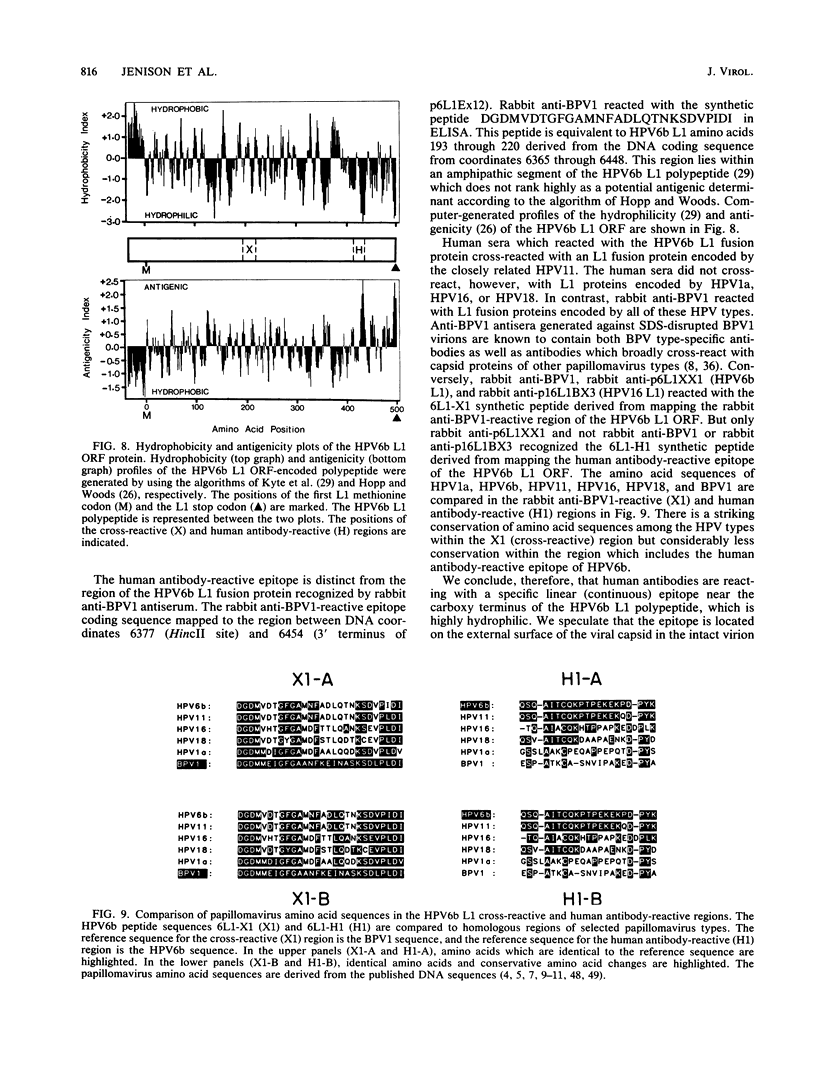
- Jenison S. A., Firzlaff J. M., Langenberg A., Galloway D. A. Identification of immunoreactive antigens of human papillomavirus type 6b by using Escherichia coli-expressed fusion proteins. J Virol. 1988 Jun;62(6):2115–2123. doi: 10.1128/jvi.62.6.2115-2123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Leure-Dupree A. E., Zaino R. J., Weber J. A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987 Feb;61(2):590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li C. C., Shah K. V., Seth A., Gilden R. V. Identification of the human papillomavirus type 6b L1 open reading frame protein in condylomas and corresponding antibodies in human sera. J Virol. 1987 Sep;61(9):2684–2690. doi: 10.1128/jvi.61.9.2684-2690.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon R. G., Sisk W., Defendi V. Expression of the E2 open reading frame of papilloma viruses BPV1 and HPV6b in Escherichia coli. Gene. 1986;42(3):241–251. doi: 10.1016/0378-1119(86)90228-3. [DOI] [PubMed] [Google Scholar]
- Mallon R. G., Wojciechowicz D., Defendi V. DNA-binding activity of papillomavirus proteins. J Virol. 1987 May;61(5):1655–1660. doi: 10.1128/jvi.61.5.1655-1660.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Mounts P., Shah K. V., Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y., Lancaster W. D., Lim L. Y., Jenson A. B. Monoclonal antibodies to genus- and type-specific papillomavirus structural antigens. Intervirology. 1986;25(1):30–37. doi: 10.1159/000149652. [DOI] [PubMed] [Google Scholar]
- Okagaki T., Clark B. A., Zachow K. R., Twiggs L. B., Ostrow R. S., Pass F., Faras A. J. Presence of human papillomavirus in verrucous carcinoma (Ackerman) of the vagina. Immunocytochemical, ultrastructural, and DNA hybridization studies. Arch Pathol Lab Med. 1984 Jul;108(7):567–570. [PubMed] [Google Scholar]
- Oriel J. D., Almeida J. D. Demonstration of virus particles in human genital warts. Br J Vener Dis. 1970 Feb;46(1):37–42. doi: 10.1136/sti.46.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel J. D. Anogenital papillomavirus infection in children. Br Med J (Clin Res Ed) 1988 May 28;296(6635):1484–1485. doi: 10.1136/bmj.296.6635.1484-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel J. D. Natural history of genital warts. Br J Vener Dis. 1971 Feb;47(1):1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass F., Maizel J. V., Jr Wart-associated antigens. II. Human immunity to viral structural proteins. J Invest Dermatol. 1973 May;60(5):307–311. doi: 10.1111/1523-1747.ep12723136. [DOI] [PubMed] [Google Scholar]
- Pfister H., zur Hausen H. Seroepidemiological studies of human papilloma virus (HPV-1) infections. Int J Cancer. 1978 Feb 15;21(2):161–165. doi: 10.1002/ijc.2910210206. [DOI] [PubMed] [Google Scholar]
- Pyrhönen S. Hunan wart-virus antibodies in patients with genital and skin warts. Acta Derm Venereol. 1978;58(5):427–432. [PubMed] [Google Scholar]
- Rando R. F., Groff D. E., Chirikjian J. G., Lancaster W. D. Isolation and characterization of a novel human papillomavirus type 6 DNA from an invasive vulvar carcinoma. J Virol. 1986 Jan;57(1):353–356. doi: 10.1128/jvi.57.1.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Schachner L., Hankin D. E. Assessing child abuse in childhood condyloma acuminatum. J Am Acad Dermatol. 1985 Jan;12(1 Pt 1):157–160. doi: 10.1016/s0190-9622(85)70020-5. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Shirasawa H., Sekine H., Simizu B. Expression of the human papillomavirus type 6b L2 open reading frame in Escherichia coli: L2-beta-galactosidase fusion proteins and their antigenic properties. Virology. 1987 May;158(1):8–14. doi: 10.1016/0042-6822(87)90231-5. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Shirasawa H., Simizu B. Expression of human papillomavirus types 6b and 16 L1 open reading frames in Escherichia coli: detection of a 56,000-dalton polypeptide containing genus-specific (common) antigens. J Virol. 1987 Aug;61(8):2389–2394. doi: 10.1128/jvi.61.8.2389-2394.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M., Gissmann L., zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981 Dec;40(3):932–935. doi: 10.1128/jvi.40.3.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]