Abstract
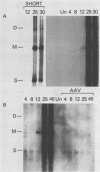
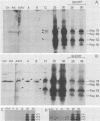
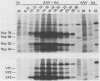
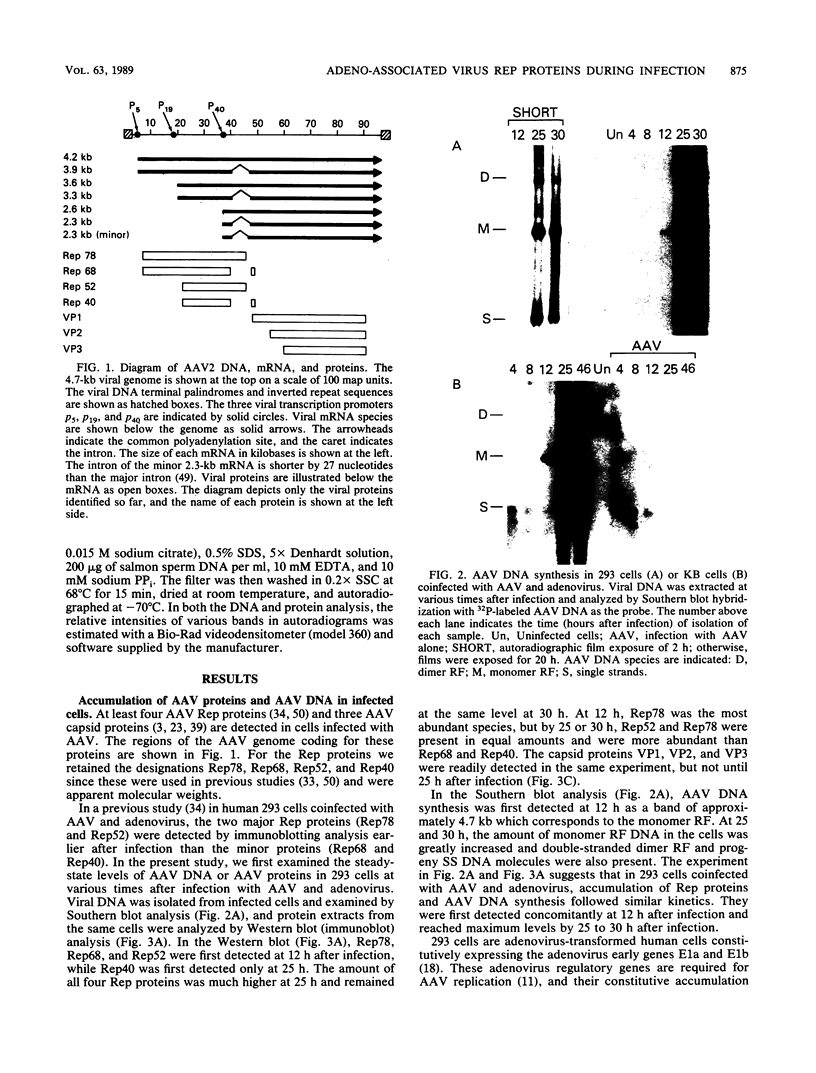
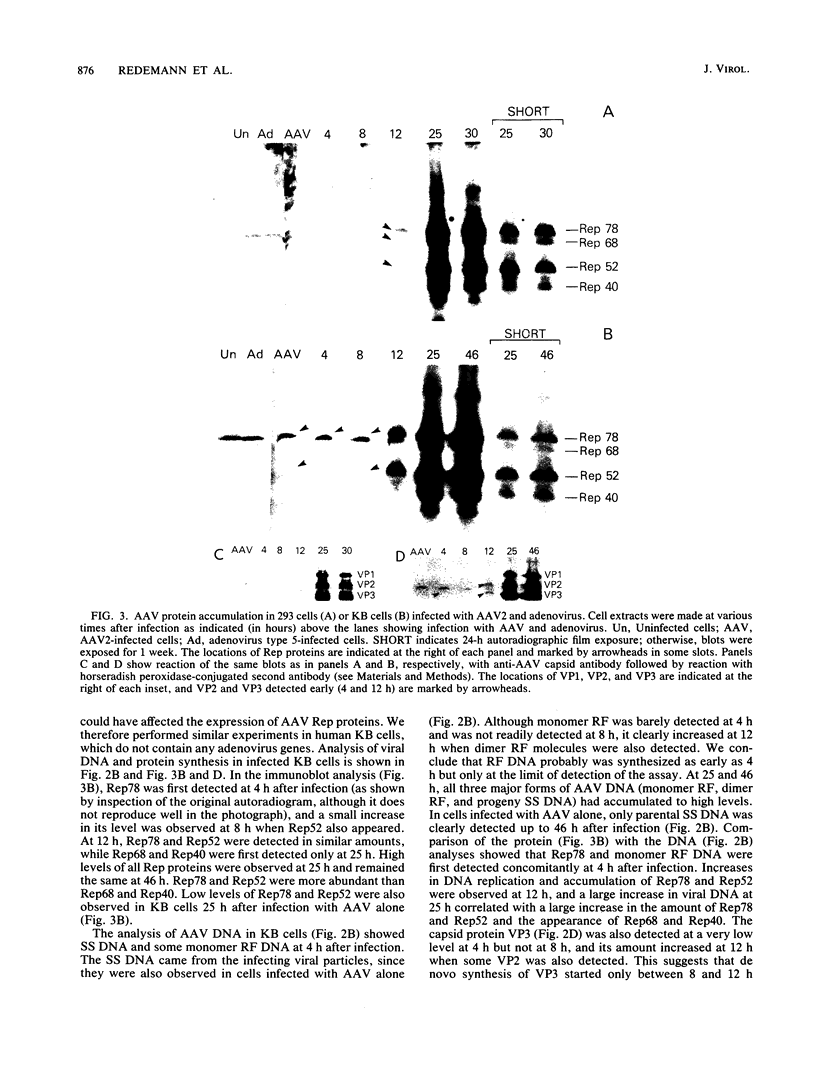
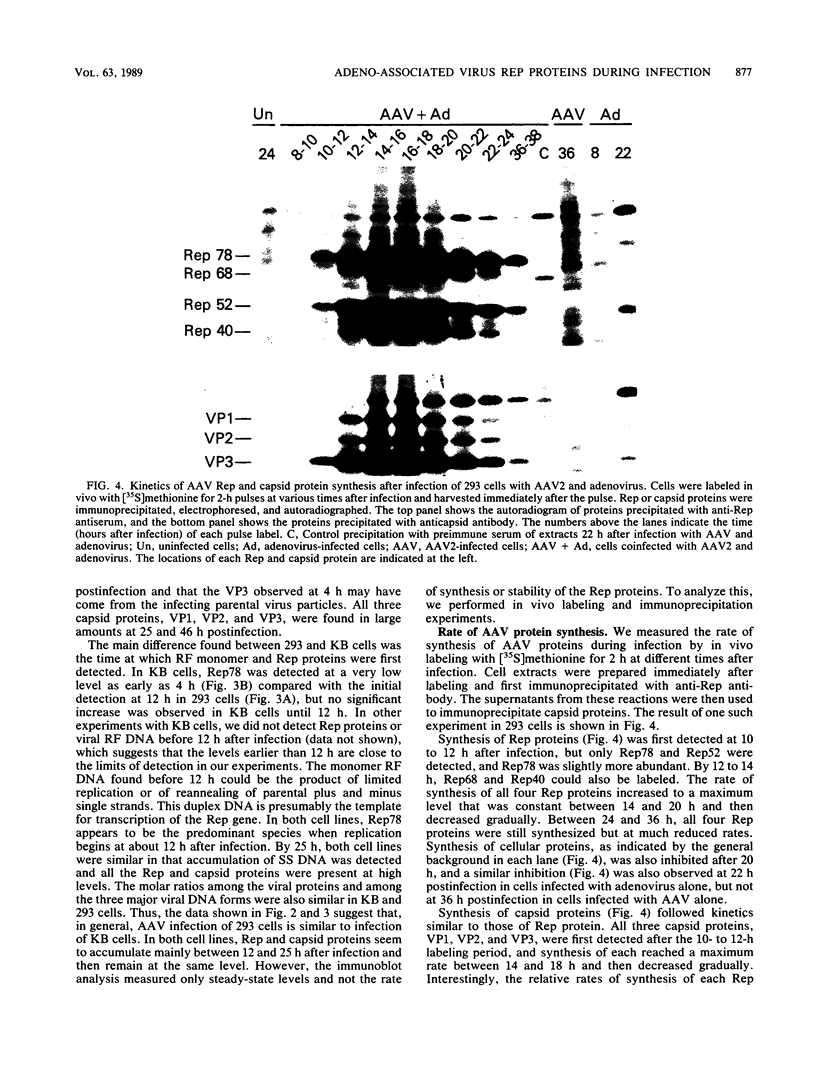
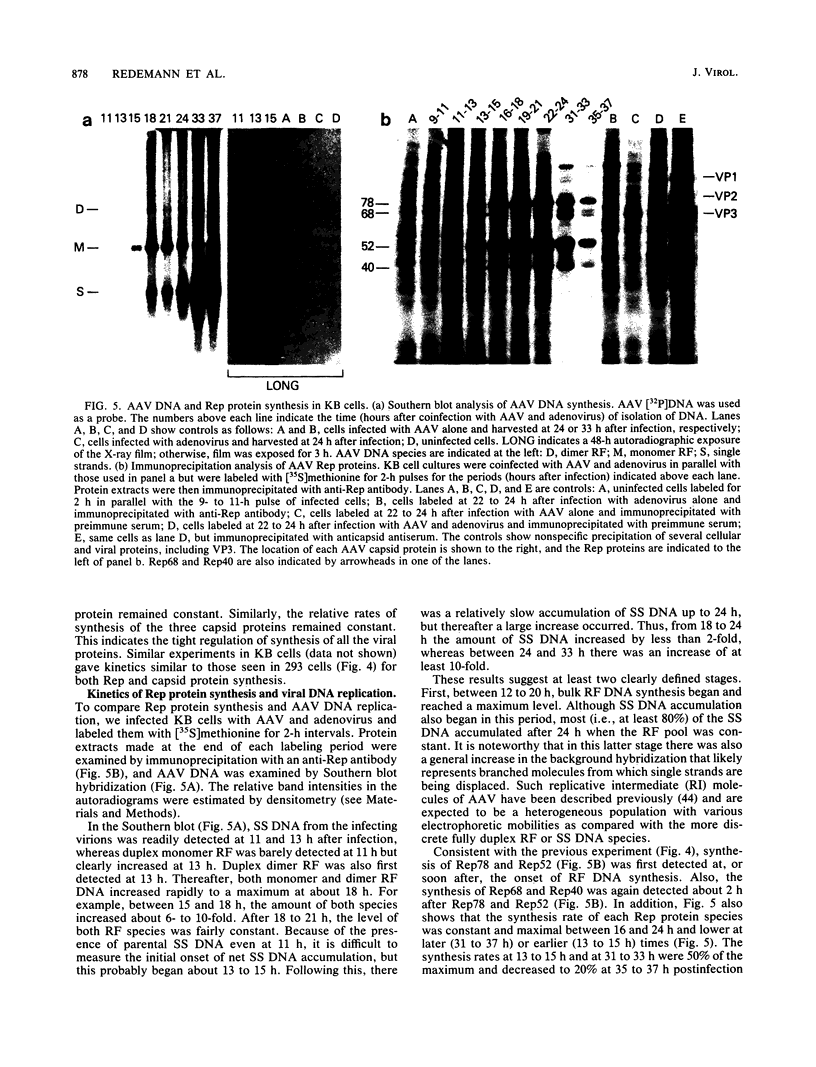
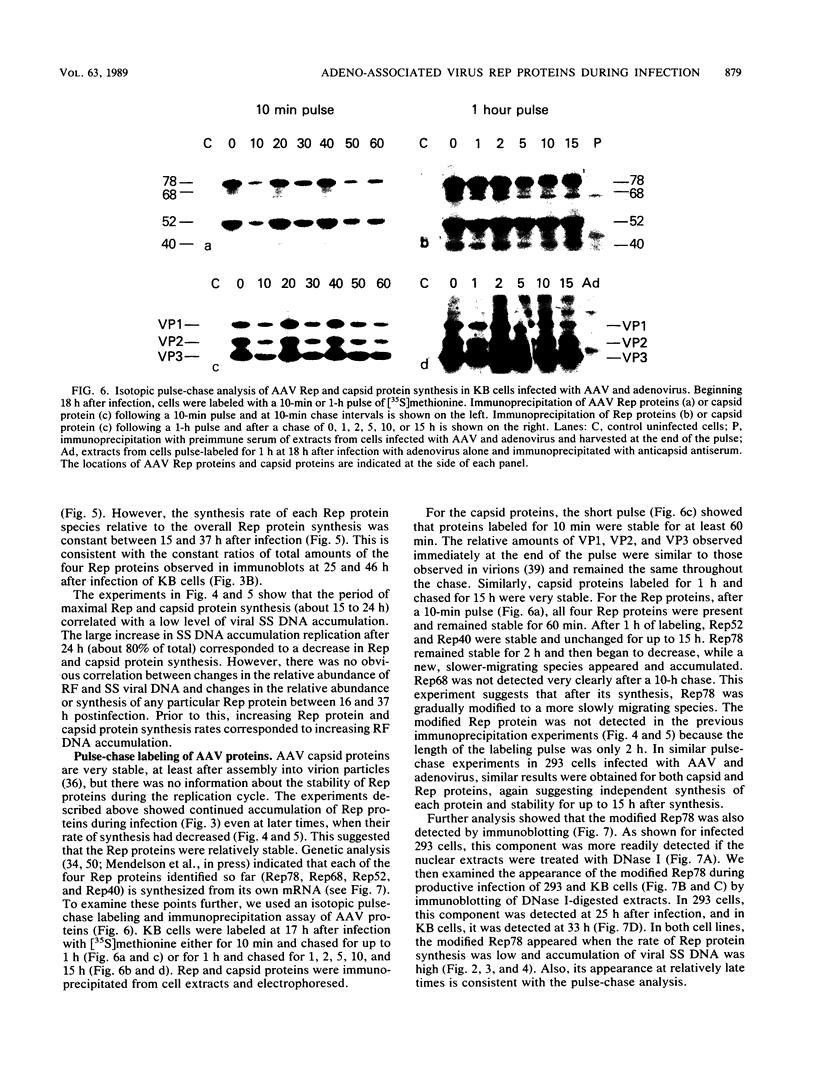
Adeno-associated virus (AAV) Rep proteins mediate viral DNA replication and can regulate expression from AAV genes. We studied the kinetics of synthesis of the four Rep proteins, Rep78, Rep68, Rep52, and Rep40, during infection of human 293 or KB cells with AAV and helper adenovirus by in vivo labeling with [35S]methionine, immunoprecipitation, and immunoblotting analyses. Rep78 and Rep52 were readily detected concomitantly with detection of viral monomer duplex DNA replicating about 10 to 12 h after infection, and Rep68 and Rep40 were detected 2 h later. Rep78 and Rep52 were more abundant than Rep68 and Rep40 owing to a higher synthesis rate throughout the infectious cycle. In some experiments, very low levels of Rep78 could be detected as early as 4 h after infection. The synthesis rates of Rep proteins were maximal between 14 and 24 h and then decreased later after infection. Isotopic pulse-chase experiments showed that each of the Rep proteins was synthesized independently and was stable for at least 15 h. A slower-migrating, modified form of Rep78 was identified late after infection. AAV capsid protein synthesis was detected at 10 to 12 h after infection and also exhibited synthesis kinetics similar to those of the Rep proteins. AAV DNA replication showed at least two clearly defined stages. Bulk duplex replicating DNA accumulation began around 10 to 12 h and reached a maximum level at about 20 h when Rep and capsid protein synthesis was maximal. Progeny single-stranded DNA accumulation began about 12 to 13 h, but most of this DNA accumulated after 24 h when Rep and capsid protein synthesis had decreased.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Becerra S. P., Koczot F., Fabisch P., Rose J. A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J Virol. 1988 Aug;62(8):2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Adler S. Separation of two types of adeno-associated virus particles containing complementary polynucleotide chains. J Virol. 1972 Feb;9(2):394–396. doi: 10.1128/jvi.9.2.394-396.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Kort J., Fife K. H., Grogan E. W., Spear I. Study of the fine structure of adeno-associated virus DNA with bacterial restriction endonucleases. J Virol. 1975 Sep;16(3):712–719. doi: 10.1128/jvi.16.3.712-719.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Pinkerton T. C., Thomas G. F., Hoggan M. D. Detection of adeno-associated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology. 1975 Dec;68(2):556–560. doi: 10.1016/0042-6822(75)90298-6. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Janik J. E., Sebring E. D., Rose J. A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981 Oct;40(1):241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Laughlin C. A., de la Maza L. M., Myers M. Adeno-associated virus autointerference. Virology. 1979 Jan 30;92(2):449–462. doi: 10.1016/0042-6822(79)90149-1. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Marcus-Sekura C. J., Laughlin C. A., Ketner G. Properties of an adenovirus type 2 mutant, Ad2dl807, having a deletion near the right-hand genome terminus: failure to help AAV replication. Virology. 1983 Apr 30;126(2):505–516. doi: 10.1016/s0042-6822(83)80008-7. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Hoggan M. D., Hauswirth W. W., Berns K. I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980 Feb;33(2):739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K. E., Pintel D. J. The two transcription units of the autonomous parvovirus minute virus of mice are transcribed in a temporal order. J Virol. 1988 Apr;62(4):1448–1451. doi: 10.1128/jvi.62.4.1448-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Establishment and characterization of KB cell lines latently infected with adeno-associated virus type 1. Virology. 1977 Oct 1;82(1):84–92. doi: 10.1016/0042-6822(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Adeno-associated virus proteins: origin of the capsid components. J Virol. 1984 Nov;52(2):591–597. doi: 10.1128/jvi.52.2.591-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Berns K. I. The adeno-associated virus rep gene inhibits replication of an adeno-associated virus/simian virus 40 hybrid genome in cos-7 cells. J Virol. 1988 May;62(5):1705–1712. doi: 10.1128/jvi.62.5.1705-1712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Hermonat P. L., Berns K. I. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J Virol. 1986 Oct;60(1):251–258. doi: 10.1128/jvi.60.1.251-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Cardellichio C. B., Coon H. C. Latent infection of KB cells with adeno-associated virus type 2. J Virol. 1986 Nov;60(2):515–524. doi: 10.1128/jvi.60.2.515-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Jones N., Carter B. J. Effect of deletions in adenovirus early region 1 genes upon replication of adeno-associated virus. J Virol. 1982 Mar;41(3):868–876. doi: 10.1128/jvi.41.3.868-876.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Westphal H., Carter B. J. Spliced adenovirus-associated virus RNA. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5567–5571. doi: 10.1073/pnas.76.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby E. W., Berns K. I. Mapping of the 5' termini of two adeno-associated virus 2 RNAs in the left half of the genome. J Virol. 1982 Feb;41(2):518–526. doi: 10.1128/jvi.41.2.518-526.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Carter B. J. Chromatin-like structure of adeno-associated virus DNA in infected cells. J Virol. 1983 Oct;48(1):79–87. doi: 10.1128/jvi.48.1.79-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C. J., Laughlin C. A., Carter B. J. Adeno-associated virus RNA transcription in vivo. Eur J Biochem. 1981 Dec;121(1):147–154. doi: 10.1111/j.1432-1033.1981.tb06443.x. [DOI] [PubMed] [Google Scholar]
- Mendelson E., Smith M. G., Miller I. L., Carter B. J. Effect of a viral rep gene on transformation of cells by an adeno-associated virus vector. Virology. 1988 Oct;166(2):612–615. doi: 10.1016/0042-6822(88)90536-3. [DOI] [PubMed] [Google Scholar]
- Mendelson E., Trempe J. P., Carter B. J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986 Dec;60(3):823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Identification and characterization of a porcine parvovirus nonstructural polypeptide. J Virol. 1985 Sep;55(3):554–559. doi: 10.1128/jvi.55.3.554-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. W., Carter B. J. Adeno-associated virus replication. The effect of L-canavanine or a helper virus mutation on accumulation of viral capsids and progeny single-stranded DNA. J Biol Chem. 1981 Jan 25;256(2):567–570. [PubMed] [Google Scholar]
- Myers M. W., Carter B. J. Assembly of adeno-associated virus. Virology. 1980 Apr 15;102(1):71–82. doi: 10.1016/0042-6822(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Maizel J. V., Jr, Inman J. K., Shatkin A. J. Structural proteins of adenovirus-associated viruses. J Virol. 1971 Nov;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987 Oct;61(10):3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Tratschin J. D., Carter B. J. Replication of adeno-associated virus DNA. Complementation of naturally occurring rep- mutants by a wild-type genome or an ori- mutant and correction of terminal palindrome deletions. J Mol Biol. 1984 Oct 15;179(1):1–20. doi: 10.1016/0022-2836(84)90303-6. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., West M. H., Sandbank T., Carter B. J. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol Cell Biol. 1984 Oct;4(10):2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Carter B. J. Alternate mRNA splicing is required for synthesis of adeno-associated virus VP1 capsid protein. J Virol. 1988 Sep;62(9):3356–3363. doi: 10.1128/jvi.62.9.3356-3363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Carter B. J. Regulation of adeno-associated virus gene expression in 293 cells: control of mRNA abundance and translation. J Virol. 1988 Jan;62(1):68–74. doi: 10.1128/jvi.62.1.68-74.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Mendelson E., Carter B. J. Characterization of adeno-associated virus rep proteins in human cells by antibodies raised against rep expressed in Escherichia coli. Virology. 1987 Nov;161(1):18–28. doi: 10.1016/0042-6822(87)90166-8. [DOI] [PubMed] [Google Scholar]
- Tullis G. E., Labieniec-Pintel L., Clemens K. E., Pintel D. Generation and characterization of a temperature-sensitive mutation in the NS-1 gene of the autonomous parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2736–2744. doi: 10.1128/jvi.62.8.2736-2744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]