Abstract
Effects of low-dose-rate gamma-irradiation on the process of tumorigenesis were investigated in mice treated with a carcinogenic agent or irradiated with high dose X-rays at a high dose rate. A prolonged gamma irradiation at approximately 1 mGy/hr suppressed the appearance of skin tumors induced by methylcholanthrene and delayed the appearance of radiation-induced thymic lymphomas in C57BL/6 mice. We also investigated the effects of low-dose-rate irradiation on disease model mice. In Type II diabetic C57BL/KsJ-db/db (db) mice, the urine glucose level was improved in some of the mice irradiated at 0.70 mGy/hr, but not in non-irradiated control mice. In MRL-lpr/lpr (lpr) mice with severe autoimmune diseases, immunological status was kept better in the mice irradiated at 0.35 or 1.2 mGy/hr. The incidence of a number of symptoms, including lymphadenopathy, splenomegaly and proteinuria, was suppressed by the irradiation. Furthermore, in both of the strains, the low-dose-rate irradiation prolonged the life span of the irradiated mice.
I. INTRODUCTION
Low doses of ionizing radiation stimulate various biological functions: anti-oxidative capacity1, DNA repair capability2, apoptosis3, 4, and immune functions5, 6. Each of these functions may work in a suppressive manner in the process of carcinogenesis, which would be initiated with DNA damage induced directly by radiation or through reactive oxygen species production. We have previously demonstrated that a low-dose-rate irradiation at around 1 mGy/hr suppressed the incidence of methylcholanthrene-induced skin tumors in ICR mice7. We also found some of biological protective functions, including anti-oxidative capacity and immune functions, were enhanced. To examine the possibility that the augmented protective capacity may have some other effects in addition to the tumor suppression, we investigated the effects of low dose irradiation on disease model mice.
II. IRRADIATION FACILITIES
Irradiation with low-dose-rate gamma rays was carried out in a clean irradiation room (Figure 1) equipped with a 137Cs gamma-ray source (370 GBq). The dose rates at 5, 7 and 10 m from the source were 1.2, 0.70 and 0.35 mGy/hr, respectively. The irradiation was continued, except for 1 hr intermissions in the morning on weekdays for care and examination of the mice. The design of the irradiation facility and the details of dosimetry with the ionization chamber and glass dosimeter have been described elsewhere8.
FIGURE 1.
Long-term low-dose-rate irradiation facility
III. EFFECTS OF LOW DOSE/DOSE-RATE IRRADIATION ON THE PROCESS OF TUMORIGENESIS
Chemically Induced Skin Tumors
Groups of thirty-five 5-week old female C57BL/6N mice were irradiated with 137Cs gamma rays at a dose rate of 0.35, 0.70 or 1.2 mGy/hr for 5 weeks. Then, to induce skin tumors, the mice were subcutaneously injected in the groin with 20-methylcholanthrene (MC), which had been dissolved in olive oil, with each mouse receiving 0.1 ml9. The dose of MC was 0.05 mg/mouse. After the MC injection, irradiation was continued at the same dose rate. All animals were given food and water ad libitum following the guidelines for animal experiments at CRIEPI.
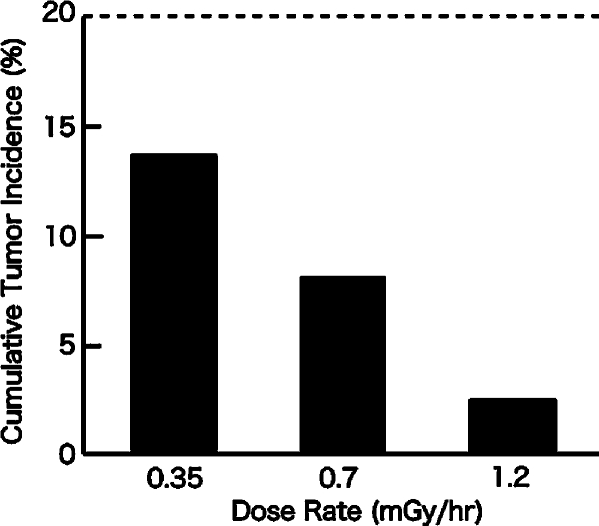
The first tumor appeared 53 days after the MC injection in non-irradiated control group. In the 0.35 mGy/hr group, the first tumors appeared 75 days after the MC injection. In the 0.70 mGy/hr group, it appeared 115 days after the injection. And in the 1.2 mGy/hr group, it appeared 108 days after the injection. Cumulative tumor incidences, 200 days after the MC injection, are shown in Figure 2.
FIGURE 2.
Cumulative tumor incidence in MC-injected mice irradiated at different dose rates. The dotted line is the cumulative tumor incidence in the non-irradiated control group.
Radiation-Induced Thymic Lymphomas
Thymic lymphomas were induced in female C57BL/6N mice by four weekly X-irradiations of 1.8 Gy each. A group of 20 female mice, 5 weeks old, were exposed to a low dose rate of 1.2 mGy/hr for 5 weeks, and then they were subjected to the repeated high dose irradiation at a high dose rate. Another group received only the fractionated high dose irradiations. The first mouse death of thymic lymphoma occurred 89 days after the last irradiation in the group that received only the repeated high dose irradiations, while 110 days elapsed before the first death in the group that received the repeated irradiations combined with the low-dose-rate irradiation. The low-dose-rate irradiation appears to have delayed the timing of the appearance of the lymphomas (Table 1).
TABLE 1.
Effects of low dose irradiation on the appearance of radiation-induced thymic lymphomas
| THYMIC LYMPHOMAS (%) | ||
|---|---|---|
| Days after 1.8 Gy irradiations | Repeated high dose irradiations only | Low-dose-rate irradiation before repeated irradiations |
| 90 | 5 | 0 |
| 100 | 20 | 0 |
| 120 | 20 | 10 |
| 140 | 45 | 25 |
| 160 | 70 | 55 |
IV. EFFECTS OF LOW-DOSE-RATE IRRADIATION ON DISEASE MODEL MICE
Type II Diabetes
A group of 12 female 10-week old C57BL/KsJ-db/db model mice with Type II diabetes mellitus10 were irradiated for life at 0.70 mGy/hr. The urine glucose levels of all of the mice were strongly positive at the beginning of the irradiation. In the irradiated group, a decrease in the glucose level was observed in three mice, one in the 35th week, another in the 52nd week and the third in the 80th week. No recovery from the diabetes was observed in the 12 mice of non-irradiated control group.
There was no systematic change of body weight or consumption of food and drinking water between the irradiated group and the non-irradiated group or between the recovered mice and the non-recovered mice.
As shown in Table 2, survival was better in the irradiated group. A marked difference was also observed in the appearance of the coat hair, skin and tail. The irradiated group was in much better condition.
TABLE 2.
Effects of Low-Dose-Rate Irradiation on Survival of C57BL/KsJ-db/db Mice
| SURVIVAL (%) | ||
|---|---|---|
| Age (weeks) | Control | Irradiated |
| 30 | 100 | 100 |
| 60 | 75 | 100 |
| 90 | 42 | 67 |
| 120 | 0 | 33 |
Mortality was delayed and the healthy appearance was prolonged in the irradiated mice by about 20–30 weeks compared with the control mice. These results suggest that the low dose irradiation modified the condition of the diabetic mice, leading not only to recovery from diabetes, but also to suppression of the aging process.
Severe Autoimmune Disease
MRL-lpr/lpr mice11 have a deletion of an apoptosis-regulating gene, Fas, that causes abnormal proliferation activity of lymphocytes, leading to severe total-body lymphadenopathy, splenomegaly and many autoimmune diseases. Groups of 12 female MRL-lpr/lpr mice, 7 weeks old, were irradiated with 137Cs gamma rays at 0.35 or 1.2 mGy/hr for 5 weeks. Then, they were kept in an animal care room without further irradiation. Table 3 summarizes the effects of low-dose-rate irradiation on the survival of the mice. The life span of MRL-lpr/lpr mice was markedly prolonged in a dose-rate dependent manner. Some of the symptoms typical in the mice with autoimmune diseases, including lymphadenopathy and proteinuria, were also suppressed12.
TABLE 3.
Effects of low-dose-rate irradiation on survival of MRL-lpr/lpr mice
| SURVIVAL (%) | |||
|---|---|---|---|
| Age (days) | 0 (mGy/hr) | 0.35 (mGy/hr) | 1.2 (mGy/hr) |
| 100 | 100 | 100 | 100 |
| 125 | 67 | 100 | 100 |
| 150 | 0 | 83 | 100 |
| 175 | 0 | 25 | 100 |
| 200 | 0 | 17 | 83 |
A cytofluorographic analysis revealed the improvement of the immunological status in the irradiated mice as the decrease in CD3+ B220+ cells, which attack their own organs and tissues, the decrease in B220+ CD40+ cells, a representative cell population in autoimmune diseases, and the increase in CD8+ T cells, which play important roles in immune activity (Table 4).
TABLE 4.
Effects of low-dose-rate irradiation on immune cell populations in MRL-lpr/lpr mice
| FRACTION OF CELL POPULATION (%) | |||
|---|---|---|---|
| Cell Population | 0 (mGy/hr) | 0.35(mGy/hr) | 1.2(mGy/hr) |
| CD3+B220+ | 42 | 36 | 24 |
| B220+CD40+ | 13 | 10 | 7.6 |
| CD8+ | 3.1 | 5.6 | 5.8 |
V. DISCUSSION
In the present study, suppression of tumorigenic processes was demonstrated (Figure 2, Table I). We have observed some increase in immune functions and tumor cell rejection capability in mice irradiated with 1.2 mGy/hr for several weeks (manuscript in preparation). Therefore, the enhancement of immune functions is presumably involved in the tumor suppression, although the contribution of other protective functions is not excluded. The significant involvement of immune functions was also demonstrated in MRL-lpr/lpr mice (Table IV).
The life span prolongation seen in the diabetic mice may not be directly correlated with the cure of diabetes, because the longer life span was seen not only in those mice showing recovery, but also in non-recovered mice. The low-dose-rate irradiation might have affected the process of aging. In the process of ageing, oxidative stress are believed to be involved.13 The increase in the antioxidative capacity in irradiated mice1, 14 might explain the anti-ageing effect of low-dose-rate irradiation.
Although their mechanisms are to be elucidated, the phenomena described in the present report should have a significant impact, on one hand, on the basic assumption in the radiological protection, which claims that radiation is harmful no matter how low the dose is. On the other hand, they would be a trigger to explore the possibility of clinical application of low dose/dose rate radiation.
REFERENCES
- 1.Kojima S., et al. Induction of mRNAs for Glutathione Synthesis-related Proteins in Mouse Liver by Low Doses of Gamma-rays. Biochim. Biophys. Acta. 1998;1384:312. doi: 10.1016/s0304-4165(98)00043-9. [DOI] [PubMed] [Google Scholar]
- 2.Le X.C., et al. Inducible Repair of Thymine Glycol Detected by an Ultrasensitive Assay for DNA Damage. Science. 1998;280:1066. doi: 10.1126/science.280.5366.1066. [DOI] [PubMed] [Google Scholar]
- 3.Cregan S.P., et al. Apoptosis and the Adaptive Response in Human Lymphocytes. Int. J. Radiat. Biol. 1999;75:1087. doi: 10.1080/095530099139548. [DOI] [PubMed] [Google Scholar]
- 4.Sakai K. Biological Effects of Low Dose Radiation, Excerpta Medica International Congress Series 1211. Elsevier; 2000. Effects of Low-Dose Preirradiation on Radiation-Induced Cell Death in Cultured Mammalian Cells; p. 53. [Google Scholar]
- 5.Nogami M., et al. Mice Chronically Exposed to Low Dose Ionizing Radiation Possess Splenocytes with Elevated Levels of HSP70 mRNA, HSC70 and HSP72 and with an Increased Capacity to Proliferate. Int. J. Radiat. Biol. 1993;63:775. doi: 10.1080/09553009314552181. [DOI] [PubMed] [Google Scholar]
- 6.Nogami M., et al. T Cells are the Cellular Target of the Proliferation-Augmenting Effect of Chronic Low-Dose Ionizing Radiation in Mice. Radiat. Res. 1994;139:48. [PubMed] [Google Scholar]
- 7.Sakai K., et al. Suppression of Carcinogenic Processes in Mice by Chronic Low Dose Rate Gamma-Irradiation. Int. J. Low Radiat. 2003;1:142. [Google Scholar]
- 8.Hoshi Y., et al. Application of a Newly Developed Photoluminescence Glass Dosimeter for Measuring the Absorbed Dose in Individual Mice Exposed to Low-Dose Rate 137Cs Gamma-Rays. J. Radiat. Res. 2000;41:129. doi: 10.1269/jrr.41.129. [DOI] [PubMed] [Google Scholar]
- 9.Tanooka H., et al. Dose Response and Growth Rates of Subcutaneous Tumors Induced with 3- Methylcholanthrene in Mice and Timing of Tumor Origin. Cancer Res. 1982;42:4840. [PubMed] [Google Scholar]
- 10.Hunnel K.P., et al. Diabetes, a New Mutation in the Mouse. Science. 1966;153:1127. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 11.Murphy D.D., Roths J.B. A Y Chromosome Associated Factor in Strain BXSB Producing Accelerated Autoimmunity and Lymphoproliferation. Arthritis Rheum. 1979;22:1188. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 12.Y. Ina and K. Sakai. Prolongation of Life Span Associated with Immunological Modification by Chronic Low-Dose-Rate Irradiation in MRL-lpr/lpr Mice. Radiat. Res. 161, In Press (2004). [DOI] [PubMed]
- 13.Finkel T., Holbrook N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature. 2000;408:239. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Nomura T., Yamaoka K. Low-Dose Gamma-Ray Irradiation Reduces Oxidative Damage Induced by CCl 4 in Mouse Liver. Free Radical Biol. Med. 1999;27:1324. doi: 10.1016/s0891-5849(99)00180-x. [DOI] [PubMed] [Google Scholar]