Abstract
PIR-A and PIR-B are activating and inhibitory Ig-like receptors on murine B lymphocytes, dendritic cells, and myeloid-lineage cells. The inhibitory function of PIR-B is mediated via its cytoplasmic immunoreceptor tyrosine-based inhibitory motifs, whereas PIR-A pairs with the Fc receptor common γ chain to form an activating receptor complex. In these studies, we observed constitutive tyrosine phosphorylation of PIR-B molecules on macrophages and B lymphocytes, irrespective of the cell activation status. Splenocyte PIR-B molecules were constitutively associated with the SHP-1 protein tyrosine phosphatase and Lyn protein tyrosine kinase. In Lyn-deficient mice, PIR-B tyrosine phosphorylation was greatly reduced. Unexpectedly, tyrosine phosphorylation of PIR-B was not observed in most myeloid and B cell lines but could be induced by ligation of the PIR molecules. Finally, the phosphorylation status of PIR-B was significantly reduced in MHC class I-deficient mice, although not in mice deficient in TAP1 or MHC class II expression. These findings suggest a physiological inhibitory role for PIR-B that is regulated by endogenous MHC class I-like ligands.
PIR-A and PIR-B are members of a subfamily of Ig-like molecules that can trigger cell activating or inhibitory signaling pathways (1–3). This subfamily includes the killer Ig-like receptors (KIR) (4–7), the p46 NK cell activating receptor (8), the Ig-like transcripts (ILT)/leucocyte Ig-like receptors (LIR)/macrophage Ig-like receptor (MIR)/monocyte cDNA 18 (9–12), and the IgA Fc receptor (FcαR) in humans (13), as well as an IgG receptor in cows (14), and the neutrophil Ig-like receptor 1 (15) and the PIR-A/B family in rats (16). PIR-A and PIR-B, which are coordinately expressed on B lymphocytes, dendritic cells, and myeloid lineage cells in the mouse (1, 3, 17), have six Ig-like domains in their extracellular regions that have similar amino acid sequences. PIR-A has a polar transmembrane region containing a charged amino acid, arginine, that is essential for its association with the Fc receptor common γ chain (FcRγc; ref. 18). This association with the immunoreceptor tyrosine-based activation motif (ITAM)-containing FcRγc is required for PIR-A cell surface expression and signal transduction (3, 17, 19–21). Conversely, PIR-B has a noncharged transmembrane region and a relatively long cytoplasmic tail containing three known immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that can recruit the SHP-1 protein tyrosine phosphatase to serve as an inhibitory receptor (3, 22, 23).
The invariant PIR-B molecule is encoded by a single gene, whereas the six or more PIR-A molecules, each having a slightly different extracellular amino acid sequence, are encoded by a multigene family (1, 3, 24, 25). The Pirb and Pira genes are located on the murine chromosome 7 in a centromeric proximal region (1, 25) that is syntenic with the human chromosome 19q13 region where genes encoding the KIR, ILT/LIR/MIR, FcαR, and p46 molecules reside (7–12). Like their KIR gene relatives, the genes encoding the PIR molecules are highly polymorphic (1, 3, 26). The MHC class I alleles that can serve as KIR ligands also are highly polymorphic, and this interaction may regulate NK cell and NK T cell activity (4–7, 27). The closest PIR relatives in humans, the ILT/LIR/MIR molecules, can also recognize MHC class I alleles (7, 10, 28, 29), whereas the ligand specificity of PIR-A and PIR-B molecules has not been defined.
In the present studies, we observed that PIR-B molecules on a variety of cell types in mice are constitutively tyrosine phosphorylated and are associated with the protein tyrosine phosphatase, SHP-1. This observation led to an examination of the association of these molecules with other candidate signaling elements in cell lines and mice with mutations in genes that could affect the PIR-B tyrosine phosphorylation status. The observation that PIR-B molecules in most cell lines were not tyrosine phosphorylated, unless ligated, suggested a role for natural PIR-B ligand(s) in this process. An analysis of the tyrosine phosphorylation status in mice deficient in MHC class II, β2 microglobulin (β2m), or TAP1 implicates nonclassical MHC class I or class I-like molecules as candidate PIR ligands.
Materials and Methods
Mice.
BALB/c (H-2d), C57BL/6 (H-2b), and C57BL/6J mice homozygous for moth-eaten viable mutation (mev/mev) were purchased from The Jackson Laboratory. Mice homozygous for targeted disruption of the Abβ gene (MHC class II−/−), β2m gene (MHC class I−/−), Fc receptor common γ chain gene (FcRγc−/−), and recombination activating gene-2 (RAG-2−/−) were purchased from Taconic Farms. Btk-deficient (Btk−/−) and TAP1-deficient (TAP1−/−) mice were purchased from The Jackson Laboratory. Lyn−/− mice were kindly provided by Takeshi Watanabe (Kyushu University, Fukuoka, Japan) (30).
Cell Preparation.
Mononuclear cell suspensions were prepared from spleen, long bones, and blood samples as described (17). Splenic macrophages were isolated by their adherence to plastic. B cells were purified from splenocyte suspensions by depletion of plastic-adherent macrophages and T cells by complement-mediated lysis initiated by treatment with anti-Thy-1 (CD90) and anti-CD4 mAbs. Purified B lymphocytes were separated into relatively small and large subpopulations by Percoll (50–85%) density gradient centrifugation.
Immunoprecipitation and Protein Blot Analysis.
Cells were solubilized in lysis buffer [1% Nonidet P-40 in 150 mM NaCl/50 mM Tris⋅HCl (pH 7.5), containing 5 mM EDTA, 20 mM iodoacetamide, 0.1% sodium azide, 20 mM ɛ-aminocaproic acid, antipain (2 μg/ml), leupeptin (1 μg/ml), soybean trypsin inhibitor (100 μg/ml), aprotinin (10 μg/ml), 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride, chymostatin (2.5 μg/ml), pepstatin A (1 μg/ml), 1 mM sodium orthovanadate, and 50 mM sodium fluoride]. Centrifuged cell lysates were analyzed by using a solid phase immunoisolation technique in which microtiter wells precoated with goat anti-rat Ig antibodies (Southern Biotechnology Associates) were incubated with the 6C1 rat anti-PIR mAb (γ1κ) or a control isotype-matched mAb before overnight incubation with the cell lysates at 4°C. Bound materials were dissociated by addition of 2% SDS, resolved on SDS/PAGE under nonreducing conditions, and transferred onto nitrocellulose membranes by electroblotting. After blocking in PBS containing 3% BSA or 7% dry milk and 0.05% Tween-20, the membranes were incubated with horseradish peroxidase (HRP)-labeled mouse anti-phosphotyrosine mAb (4G10 clone; Upstate Biotechnology, Lake Placid, NY) or rabbit antibodies specific either for protein tyrosine phosphatase SHP-1 (Upstate Biotechnology) or SHP-2 (Santa Cruz Biotechnology). HRP-labeled goat anti-rabbit Ig antibody (0.1 μg/ml; Southern Biotechnology Associates) was used as the developing reagent for rabbit antibodies before visualization by enhanced chemiluminescence (Amersham). The membranes were reblotted with rabbit anti-PIR antiserum (1:4,000 dilution) to identify the PIR molecules. The levels of PIR-B tyrosine phosphorylation in mice with various gene mutations relative to those in wild-type control mice were determined by densitometric analysis. In brief, the relative intensities of PIR-B bands for wild-type vs. mutant mice on the same exposed membranes immunoblotted with anti-phosphotyrosine (anti-PY) and anti-PIR antibodies were measured by a Fluor-S MultiImager instrument (Bio-Rad, Hercules, CA). The relative level of PIR-B tyrosine phosphorylation was determined by: [(PIR-B intensity for anti-PY blots in mutant mice)/(PIR-B intensity for anti-PIR blots in mutant mice) ÷ (PIR-B intensity for anti-PY blots in wild-type mice)/(PIR-B intensity for anti-PIR blots in wild-type mice)] ×100. Statistical evaluation was performed by using a T test with the null hypothesis that the value of 100 would indicate no difference of PIR-B intensity between the wild-type and mutant mice.
Other reagents used for immunoblotting included rabbit antibodies specific for Btk, Cbl, Lyn, PLCγ2, SHIP (Santa Cruz), PI3 kinase, Vav (Upstate Biotechnology), FcRγc (a gift from Jeffery C. Edberg, University of Alabama at Birmingham) (31), BLNK (a gift from Andrew C-Y. Chan, Washington University Medical Institute) (32), DAP12 (a gift from Kerry S. Campbell, Fox Chase Cancer Center) (33, 34), or SLP-65 (a gift of Michael Reth, Max Planck Institute, Freiberg) (35), a mouse mAb against casein kinase IIα (Transduction Laboratories, Lexington, KY), and HRP-labeled goat anti-mouse Ig antibody (Southern Biotechnology Associates).
Results
Constitutive Tyrosine Phosphorylation of PIR-B on Normal B Lymphocytes and Myeloid Lineage Cells.
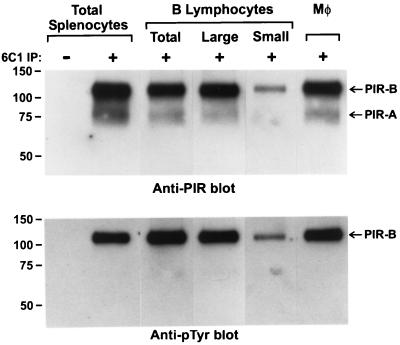
When PIR molecules were isolated from the splenocyte population and examined by immunoblotting with an anti-phosphotyrosine mAb, PIR-B was found to be constitutively phosphorylated (Fig. 1). PIR-A and PIR-B molecules are normally expressed on a variety of cell types, including the B lymphocytes, macrophages, neutrophils, and dendritic cells in the spleen. When splenic B lymphocytes, the predominant cell type, and macrophages were purified, their examination indicated constitutive tyrosine phosphorylation of the PIR molecules on both cell types. Because splenic B cells represent a heterogeneous population ranging from small resting lymphocytes to relatively large, activated lymphocytes, these two major subpopulations were examined after their separation by density gradient centrifugation. Analysis of the small and large B lymphocyte subpopulations indicated that the PIR-B molecules on both types of B cells are constitutively tyrosine phosphorylated (Fig. 1). In addition, the PIR-B molecules on granulocytes in blood and bone marrow and on macrophages in peritoneal exudates also were found to be constitutively tyrosine phosphorylated (data not shown). These results indicate that the PIR-B molecules on murine cells that coordinately express the paired Ig-like receptors, PIR-A and PIR-B, are constitutively tyrosine phosphorylated.
Figure 1.
Analysis of the tyrosine phosphorylation status of PIR-B molecules expressed by splenic cells. B cells were purified from splenocyte suspensions by depletion of plastic adherent cells and of the T cells by complement-mediated lysis initiated by anti-CD90 and anti-CD4 treatment and further separated into relatively large and small subpopulations by Percoll gradient centrifugation. Splenic macrophages were purified by adhesion to plastic Petri dishes. Aliquots of various populations were lysed and the anti-PIR immunoprecipitates separated by SDS/PAGE before transfer onto nylon filters and immunoblotting. The bands were developed with secondary HRP-labeled antibodies and revealed by enhanced chemiluminescence.
Identification of Molecules Associated with PIR-A and PIR-B.
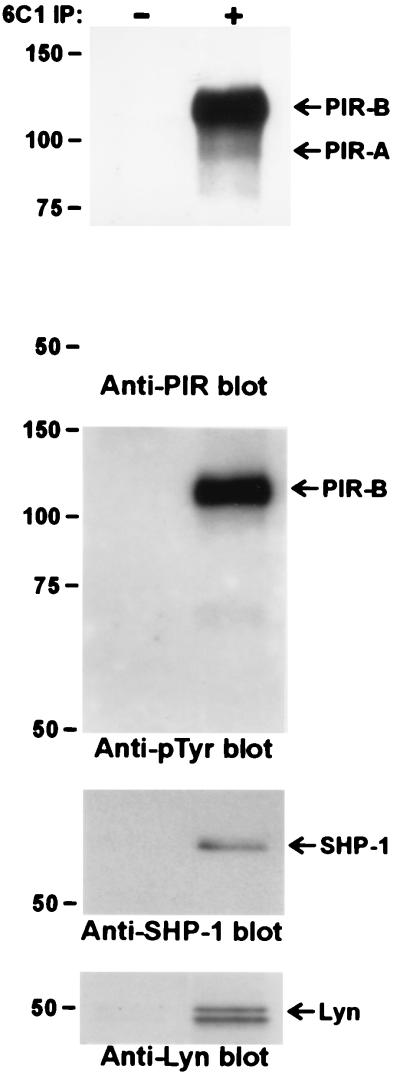
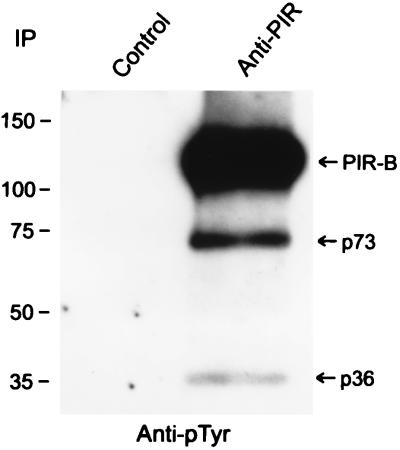
The 6C1 mAb, which reacts with a nonallelic extracellular epitope common to both PIR-A and PIR-B molecules, was used to immunoprecipitate the PIR molecules in experiments designed to identify associated molecules. These anti-PIR immunoprecipitates were consistently found to contain SHP-1 protein tyrosine phosphatase and Lyn protein tyrosine kinase (Fig. 2). Two additional tyrosine-phosphorylated proteins of ≈73 and ≈36 kDa were observed in the anti-PIR immunoprecipitates (Fig. 3), although the identity of these molecules was not revealed in the present studies. Molecules that were specifically sought in the anti-PIR immunoprecipitates, but which could not be identified, included SHP-2, SHIP, Btk, PI3-kinase, PLCγ2, casein kinase IIα, Cbl, Vav, SLP-65/BLNK, and DAP12.
Figure 2.
Constitutive in vivo association of PIR-B with SHP-1 and Lyn. Freshly isolated splenocytes were lysed and the anti-PIR immunoprecipitates analyzed as in Fig. 1.
Figure 3.
Constitutive association of PIR molecules with other tyrosine-phosphorylated molecules. Anti-PIR immunoprecipitates of lysed splenocytes were analyzed by immunoblotting with an anti-phosphotyrosine antibody as in Fig. 1, except for a longer chemiluminescence exposure time.
Analysis of Mutant Mice to Identify Participants in the PIR-B Tyrosine Phosphorylation Process.
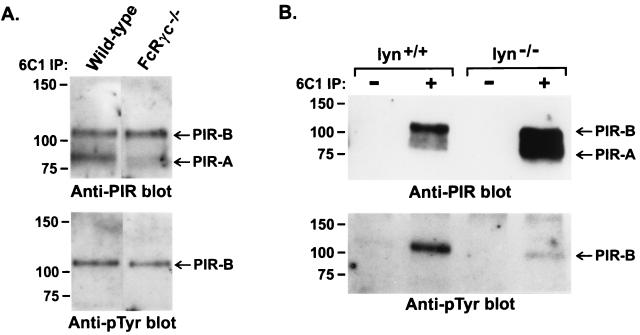
The coordinate expression of PIR-A and PIR-B by cells of myeloid and B cell lineages contributed to their being named paired Ig-like receptors (1). This feature, plus the association of cell surface PIR-A with the ITAM-bearing FcRγc signaling element (17, 19–21), raised the possibility that the PIR-A/FcRγc complex might activate protein tyrosine kinases which in turn phosphorylate the PIR-B molecules. To test this possibility, splenocytes were examined from FcRγc−/− mice that fail to express cell surface PIR-A receptors (17). This analysis indicated a normal PIR-B tyrosine phosphorylation status for splenocytes from the FcRγc-deficient mice (Fig. 4A), suggesting that the PIR-A/FcRγc complex is not required for PIR-B phosphorylation. In contrast, the level of PIR-B tyrosine phosphorylation was found to be reduced by >90% when splenocytes from Lyn-deficient mice were examined (Fig. 4B). The Src family member, Lyn, thus appears to be a major participant in the constitutive tyrosine phosphorylation of PIR-B molecules.
Figure 4.
Analysis of PIR-B tyrosine phosphorylation in FcRγ−/− (A) and Lyn−/− (B) mice. Anti-PIR immunoprecipitates of lysed splenocytes from the indicated mouse strains were examined as in Fig. 1.
Analysis of the inhibitory potential of PIR-B has indicated the association of SHP-1 protein tyrosine phosphatase with tyrosine-phosphorylated ITIMs in PIR-B (22, 23). When the PIR-B tyrosine phosphorylation status was examined in splenocytes from moth-eaten viable mice (mev/mev) deficient in SHP-1 activity (36, 37), however, the tyrosine phosphorylation status of PIR-B was not compromised. PIR-B molecules also were found to be normally tyrosine phosphorylated in Btk−/−, FcRγc−/−, and RAG-2−/− mice (data not shown).
Constitutive Tyrosine Phosphorylation of PIR-B is Not the Rule in Cell Lines.
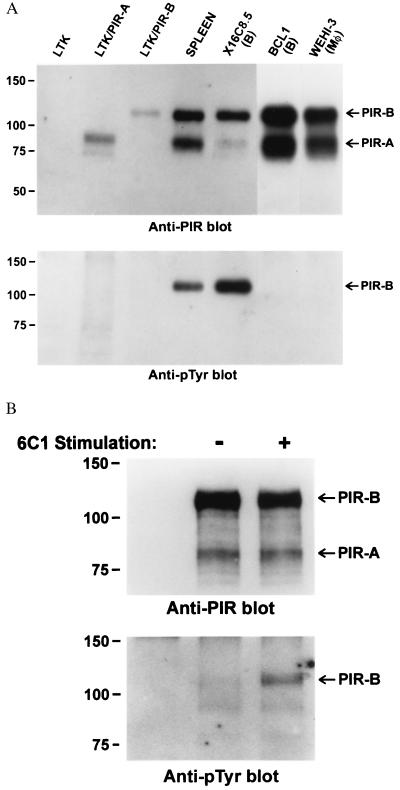
The finding of constitutive tyrosine phosphorylation of PIR-B molecules in mice led us to examine the PIR-B tyrosine phosphorylation status in cell lines representative of in vivo hematopoietic lineages that possess tyrosine-phosphorylated PIR-B molecules. These included B cell, macrophage, and mast cell lines. In addition, the LTK fibroblast cell line was examined before and after transduction of PIR-A or PIR-B expression. Remarkably, the PIR-B molecules in the cell lines were rarely found to be constitutively tyrosine phosphorylated (Fig. 5A). An exception to this rule was the X16C8.5 B cell line, which could easily be shown to express tyrosine-phosphorylated PIR-B molecules. This cell line, which expressed relatively low levels of PIR-A molecules, was noted to grow in dense cell clusters in contrast with the other cell lines. However, increasing the cell density of the other PIR-A/PIR-B positive cell lines failed to enhance their PIR-B tyrosine phosphorylation status.
Figure 5.
(A) Analysis of the tyrosine phosphorylation status of PIR-B molecules expressed by B lymphocyte, myeloid, and transfected fibroblast cell lines. Cultured cells representative of B lymphocytes (X16C8.5 and BCL-1), macrophages (WEHI-3), and transfected fibroblasts (LTK) were lysed and their PIR-B molecules examined for tyrosine phosphorylation as in Fig. 1. (B) Analysis of the PIR-B tyrosine phosphorylation status in the WEHI-3 macrophage cell line before and after PIR ligation with the 6C1 anti-PIR antibody. Cells were lysed for analysis after 2-min incubation with the 6C1 anti-PIR antibody or a control antibody.
Ligation Induced PIR-B Tyrosine Phosphorylation in the WEHI-3 Cell Line.
A Pirb gene mutation could have explained the lack of tyrosine phosphorylation of the PIR-B molecules expressed by the cell lines. However, cloning and sequencing of PIR-B transcripts in WEHI-3 cells revealed a normal cDNA sequence. In addition, the PIR-B molecules on these cells were of normal size (Fig. 5A) and immunofluorescence analysis with anti-PIR mAbs indicated normal cell surface expression.
To examine the possibility that cross-linkage of the PIR molecules can induce PIR-B tyrosine phosphorylation, the WEHI-3 cells were incubated with the 6C1 anti-PIR mAb before analysis of the PIR-B tyrosine phosphorylation status. In these experiments, ligation of the PIR molecules was observed to result in a prompt induction of PIR-B tyrosine phosphorylation; e.g., a sevenfold increase by 2 min can be seen in the experiment illustrated in Fig. 5B. In addition to indicating the integrity of PIR-B tyrosine phosphorylation mechanisms in WEHI-3 cells, this analysis indicates that tyrosine phosphorylation of the PIR-B molecules is not an inherent characteristic of their cell surface expression and may require a ligand-mediated signal for its induction.
Analysis of the PIR-B Tyrosine Phosphorylation Status in MHC-Deficient Mice.
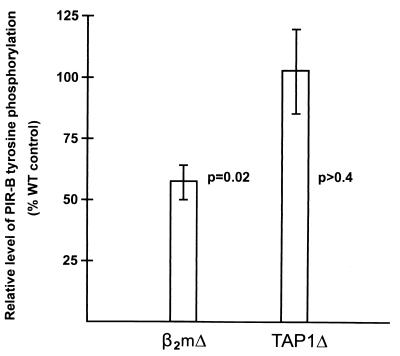
The above results imply that the PIR-B molecules are constitutively tyrosine phosphorylated as a consequence of interaction with self ligands. One of the PIR relatives is the FcαR α chain that binds IgA, but our earlier studies failed to reveal the capacity for PIR-B to bind IgM, IgG, or IgA (Toshihide Shimada and H.K., unpublished observations). Members of the KIR and ILT/LIR/MIR families, closer human relatives of the murine PIRs, have been shown to bind classical or nonclassical MHC class I alleles (7, 10, 28, 29), thus suggesting the possibility that the PIR ligands also could be MHC class I molecules. As an initial step in examining this hypothesis, the PIR-B tyrosine phosphorylation status was examined in β2m−/−, TAP1−/−, and MHC class II−/− mice. This analysis indicated that the level of PIR-B tyrosine phosphorylation is reduced by ≈50% in β2m-deficient mice but is not significantly altered in TAP1-deficient mice (Fig. 6) or MHC class II-deficient mice (data not shown).
Figure 6.
Analysis of PIR-B tyrosine phosphorylation status in mice with β2m deficiency (β2mΔ) or TAP-1 deficiency (TAP1Δ). The relative levels of PIR-B tyrosine phosphorylation (mean ± 1 SE) were determined for seven experimental animals in each group as described in the Materials and Methods.
Discussion
PIR-A and PIR-B are paired “on” and “off” receptors that may participate in regulating the activation status of B lymphocytes, dendritic cells, and myeloid cells. The present studies unexpectedly indicated constitutive tyrosine phosphorylation of the PIR-B molecules irrespective of cell type and activation status. The latter conclusion was evidenced by constitutive tyrosine phosphorylation of PIR-B molecules in small resting B lymphocytes as well as in the activated, relatively large B cells in the mouse spleen.
Earlier studies indicate that the SHP-1 protein tyrosine phosphatase can associate with the tyrosine-phosphorylated ITIMs in PIR-B to inhibit cell activation pathways (3, 22, 23). The present studies indicate that at least four molecules are constitutively associated with the native PIR molecules: SHP-1, Lyn protein tyrosine kinase, and two tyrosine-phosphorylated molecules of ≈73 and ≈36 kDa (Fig. 4), the nature and physiological roles of which remain to be elucidated. Remarkably, the level of PIR-B tyrosine phosphorylation was found to be reduced by >90% in Lyn-deficient mice, thereby inferring that Lyn is the major participant in the constitutive tyrosine phosphorylation of PIR-B.
In contrast with the constitutive tyrosine phosphorylation observed for PIR-B molecules in vivo, this was not found to be the rule in cell lines that express PIR-A and PIR-B. When the WEHI-3 myeloid cell line was examined in more detail, these cells were found to express an abundance of PIR-A and nontyrosine-phosphorylated PIR-B molecules of normal size and distribution. In addition, a cDNA sequence analysis revealed no PIR-B mutations. These results indicated that cell surface expression of the PIR-B molecules per se is insufficient for their tyrosine phosphorylation. The possibility of a PIR ligation requirement was supported by the demonstration that PIR-B tyrosine phosphorylation could be induced by treatment of the WEHI-3 cells with bivalent anti-PIR antibodies. Collectively, these observations suggest that the constitutive activation of inhibitory PIR-B molecules is initiated by interaction with self-ligand(s).
The PIR-A and PIR-B members comprise a family of polymorphic cell surface receptors in mice that are close relatives of the human ILT/LIR/MIR and KIR/KAR families, members of which have been shown to have binding specificity for MHC class I alleles (4–7, 10, 27–29), and the human FcαR, which binds IgA (13). Because our previous studies of PIR-B-transfected fibroblasts failed to reveal IgA, IgG, or IgM binding, this result led to the examination of the PIR-B tyrosine phosphorylation status in mutant mice with different MHC deficiencies. In this analysis, the PIR-B tyrosine phosphorylation level was found to be significantly reduced in β2m-deficient mice, thereby providing indirect evidence for a MHC class I or class I-like ligand. However, the finding of a normal status of PIR-B tyrosine phosphorylation in TAP1-deficient mice questions the role of classical MHC class I molecules as natural PIR ligands because TAP1 is required for peptide loading and stable cell surface expression of these molecules (38). Nonclassical MHC class I or class I-like molecules (39, 40) therefore appear to be more likely candidates for native PIR ligands. In this regard, it is noteworthy that, although the compromised tyrosine phosphorylation of PIR-B observed in β2m−/− mice clearly implies a physiological role for β2m-containing molecules, the PIR-B tyrosine phosphorylation level in the mutant mice was reduced only by ≈50%. This clue and the sequence variability of PIR-A molecules (1, 26) suggest that future ligand fishing experiments could yield multiple PIR ligands.
Acknowledgments
We thank Drs. Jeffrey V. Ravetch and Eric Vivier for critical comments on the manuscript and Charles Mashburn, Marsha Flurry, Dottie Lang, and E. Ann Brookshire for help in manuscript preparation. This work has been supported in part by National Institutes of Health Grants AI39816 and AI42127. M.D.C. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- PIR
paired Ig-like receptors
- KIR
killer cell inhibitory receptors
- ILT
Ig-like transcripts
- LIR
leukocyte Ig-like receptors
- MIR
monocyte/macrophage Ig-like receptors
- FcαR
Fc receptor for IgA
- FcRγc
Fc receptor common γ chain
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- HRP
horseradish peroxidase
- β2m
β2 microglobulin
References
- 1.Kubagawa H, Burrows P D, Cooper M D. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayami K, Fukuta D, Nishikawa Y, Yamashita Y, Inui M, Ohyama Y, Hikida M, Ohmori H, Takai T. J Biol Chem. 1997;272:7320–7327. doi: 10.1074/jbc.272.11.7320. [DOI] [PubMed] [Google Scholar]
- 3.Kubagawa H, Cooper M D, Chen C C, Ho L H, Alley T L, Hurez V, Tun T, Uehara T, Shimada T, Burrows P D. Curr Top Microbiol Immunol. 1999;244:137–149. doi: 10.1007/978-3-642-58537-1_12. [DOI] [PubMed] [Google Scholar]
- 4.Colonna M, Samaridis J. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 5.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari M C, Moretta L. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 6.Lanier L L. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 7.Long E O. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 8.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samaridis J, Colonna M. Eur J Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 10.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu M L. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 11.Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long E O. Curr Biol. 1997;7:615–618. doi: 10.1016/s0960-9822(06)00263-6. [DOI] [PubMed] [Google Scholar]
- 12.Arm J P, Nwankwo C, Austen K F. J Immunol. 1997;159:2342–2349. [PubMed] [Google Scholar]
- 13.Maliszewski C R, March C J, Schoenborn M A, Gimpel S, Shen L. J Exp Med. 1990;172:1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G, Young J R, Tregaskes C A, Sopp P, Howard C J. J Immunol. 1995;155:1534–1541. [PubMed] [Google Scholar]
- 15.Berg S F, Fossum S, Dissen E. Eur J Immunol. 1999;29:2000–2006. doi: 10.1002/(SICI)1521-4141(199906)29:06<2000::AID-IMMU2000>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Dennis, G., Stephan, R. P., Kubagawa, H. & Cooper, M. D. (1999) J. Immunol., 163, in press. [PubMed]
- 17.Kubagawa H, Chen C C, Ho L H, Shimada T, Gartland L, Mashburn C, Uehara T, Ravetch J V, Cooper M D. J Exp Med. 1999;189:309–317. doi: 10.1084/jem.189.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravetch J V, Kinet J P. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 19.Maeda A, Kurosaki M, Kurosaki T. J Exp Med. 1998;188:991–995. doi: 10.1084/jem.188.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita Y, Ono M, Takai T. J Immunol. 1998;161:4042–4047. [PubMed] [Google Scholar]
- 21.Taylor L S, McVicar D W. Blood. 1999;94:1790–1796. [PubMed] [Google Scholar]
- 22.Bléry M, Kubagawa H, Chen C C, Vély F, Cooper M D, Vivier E. Proc Natl Acad Sci USA. 1998;95:2446–2451. doi: 10.1073/pnas.95.5.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. J Exp Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alley T L, Cooper M D, Chen M, Kubagawa H. Tissue Antigens. 1998;51:224–231. doi: 10.1111/j.1399-0039.1998.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita Y, Fukuta D, Tsuji A, Nagabukuro A, Matsuda Y, Nishikawa Y, Ohyama Y, Ohmoi H, Ono M, Takai T. J Biochem (Tokyo) 1998;123:358–368. doi: 10.1093/oxfordjournals.jbchem.a021945. [DOI] [PubMed] [Google Scholar]
- 26.Chen C C, Hurez V, Brockenbrough J S, Kubagawa H, Cooper M D. Proc Natl Acad Sci USA. 1999;96:6868–6872. doi: 10.1073/pnas.96.12.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanier L L. Cell. 1998;92:705–707. doi: 10.1016/s0092-8674(00)81398-7. [DOI] [PubMed] [Google Scholar]
- 28.Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. J Exp Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges L, Hsu M L, Fanger N, Kubin M, Cosman D. J Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 30.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 31.Edberg J C, Yee A M F, Rakshit D S, Chang D J, Gokhale J A, Indik Z K, Schreiber A D, Kimberly R P. J Biol Chem. 1999;274:30328–30333. doi: 10.1074/jbc.274.42.30328. [DOI] [PubMed] [Google Scholar]
- 32.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan A C, Kurosaki T. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 33.Campbell K S, Cella M, Carretero M, Lopez-Botel M, Colonna M. Eur J Immunol. 1998;28:599–609. doi: 10.1002/(SICI)1521-4141(199802)28:02<599::AID-IMMU599>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Campbell K S, Colonna M. Int J Biochem Cell Biol. 1999;31:631–636. doi: 10.1016/s1357-2725(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 35.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen P J, Reth M. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlowski M, Mlinaric-Rascan I, Feng G S, Shen R, Pawson T, Siminovitch K A. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsui H W, Siminovitch K A, deSouza L, Tsui F W L. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 38.Pamer E, Cresswell P. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 39.Vitale M, Castriconi R, Parolini S, Pende D, Hsu M L, Moretta L, Cosman D, Moretta A. Int Immunol. 1999;11:29–35. doi: 10.1093/intimm/11.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Allan D S J, Colonna M, Lanier L, Churakova T D, Abrams J S, Ellis S A, McMichael A J, Braud V M. J Exp Med. 1999;189:1149–1155. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]