Abstract
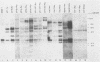
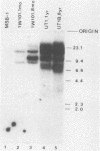
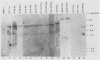
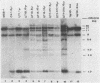
The expression of the v-rel oncogene of avian reticuloendotheliosis virus (REV-T) transforms and immortalizes very immature avian lymphoid cells. In REV-T-transformed lymphoid cells which were persistently infected with reticuloendotheliosis-associated virus (REV-A), the REV-T proviral copy number increases after the initial integration event. In 23 independently derived REV-T-transformed cell lines, 15 of the 18 virus-producing cell lines had acquired additional proviruses. The rate at which the newly acquired proviral sequences accumulated differed for various cell lines. In some cell lines, additional REV-T proviral copies could be detected as early as 8 months after the initial integration event. A correlation exists between the number of REV-T proviral sequences and the length of time which a given cell line had been propagated in culture. The integration sites occupied by the newly acquired REV-T proviruses were distinct. In contrast, reticuloendotheliosis-associated virus proviral sequences in these REV-T-transformed virus-producing lymphoid cells did not increase during in vitro culture. Furthermore, the acquisition of additional REV-T proviral sequences did not occur in non-virus-producing cell lines. Two of the newly acquired proviral sequences were molecularly cloned and analyzed by restriction endonuclease mapping. Although the newly acquired REV-T proviruses have not sustained major deletions, the viral sequences and the v-rel oncogene display numerous restriction enzyme polymorphisms. The cellular flanking sequences of two newly acquired REV-T proviruses analyzed were unique and shared no homology with flanking sequences of the other REV-T proviruses in these transformed cells. The nucleotide sequence of the virus-cellular DNA junctions of one newly acquired provirus and its cellular sequence prior to proviral integration were defined. A 5-base-pair direct repeat of cellular origin was present on each side of the long terminal repeat, indicating that the mechanism of acquisition of additional REV-T proviral sequences used reverse transcription and integration of new REV-T proviral copies.
Full text
PDF



Images in this article
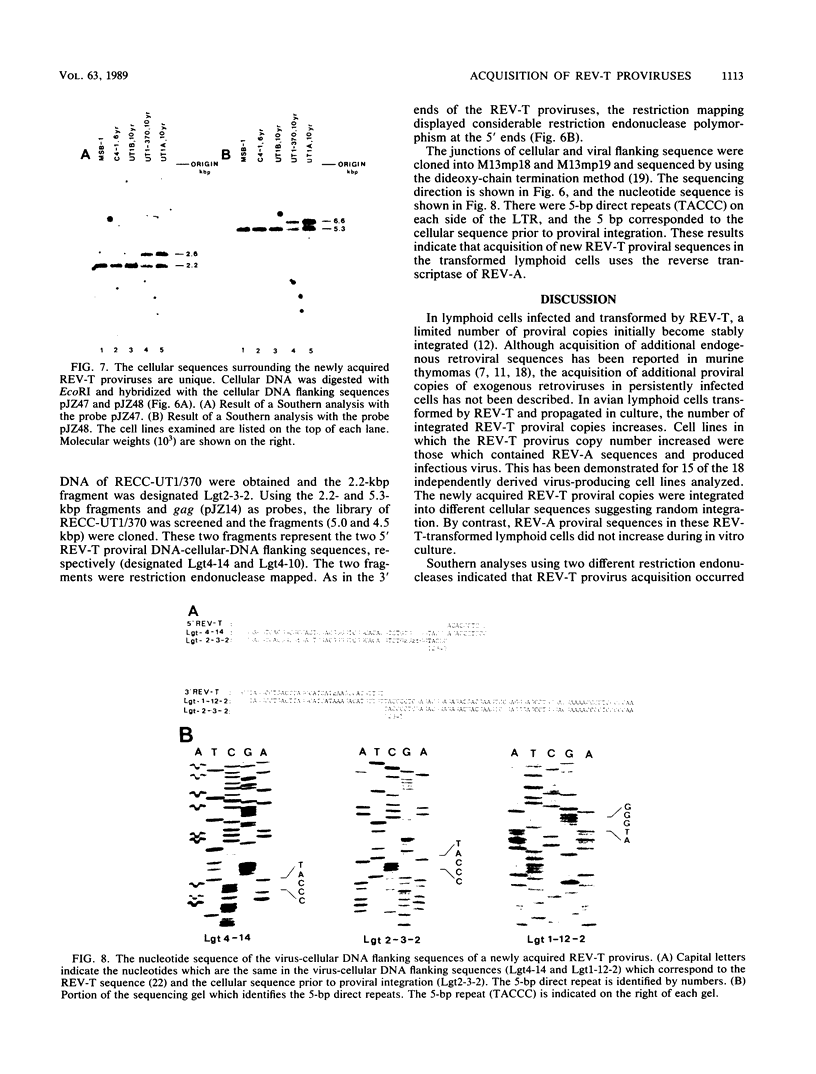
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Mak T. W., O'Rear J. J., Temin H. M. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981 Dec;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Substitution of 5' helper virus sequences into non-rel portion of reticuloendotheliosis virus strain T suppresses transformation of chicken spleen cells. Cell. 1982 Nov;31(1):111–120. doi: 10.1016/0092-8674(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Wilhelmsen K. C., Temin H. M. Structure and expression of c-rel, the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1983 Jan;45(1):104–113. doi: 10.1128/jvi.45.1.104-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lim M. Y., Bose H., Jr, Bishop J. M. Rearrangements of chicken immunoglobulin genes in lymphoid cells transformed by the avian retroviral oncogene v-rel. Proc Natl Acad Sci U S A. 1988 Jan;85(2):549–553. doi: 10.1073/pnas.85.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J., Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984 Jan;49(1):92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Cell killing by spleen necrosis virus is correlated with a transient accumulation of spleen necrosis virus DNA. J Virol. 1979 Aug;31(2):376–388. doi: 10.1128/jvi.31.2.376-388.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Ikuta K., Kato S. Detection of T-cell surface determinants in three Marek's disease lymphoblastoid cell lines. Biken J. 1976 Mar;19(1):29–32. [PubMed] [Google Scholar]
- Rice N. R., Hiebsch R. R., Gonda M. A., Bose H. R., Jr, Gilden R. V. Genome of reticuloendotheliosis virus: characterization by use of cloned proviral DNA. J Virol. 1982 Apr;42(1):237–252. doi: 10.1128/jvi.42.1.237-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Chen I., Howatson A., Mak T. W. Morphological, immunological, and biochemical analyses of chicken spleen cells transformed in vitro by reticuloendotheliosis virus strain T. Cancer Res. 1982 Jul;42(7):2722–2728. [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Allen P. T. RNA-directed DNA polymerase activity of reticuloendotheliosis virus: characterization of the endogenous and exogenous reactions. J Virol. 1975 Oct;16(4):872–879. doi: 10.1128/jvi.16.4.872-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]