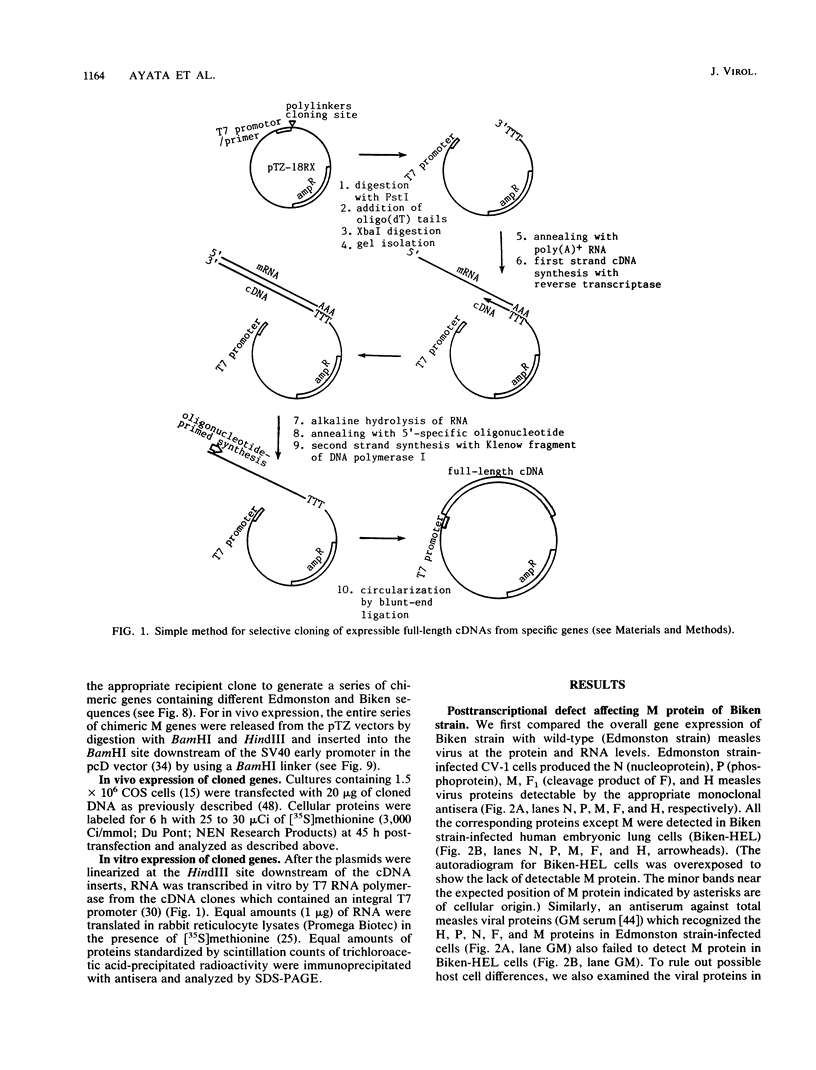
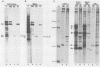Abstract
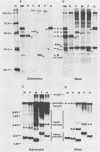
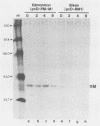
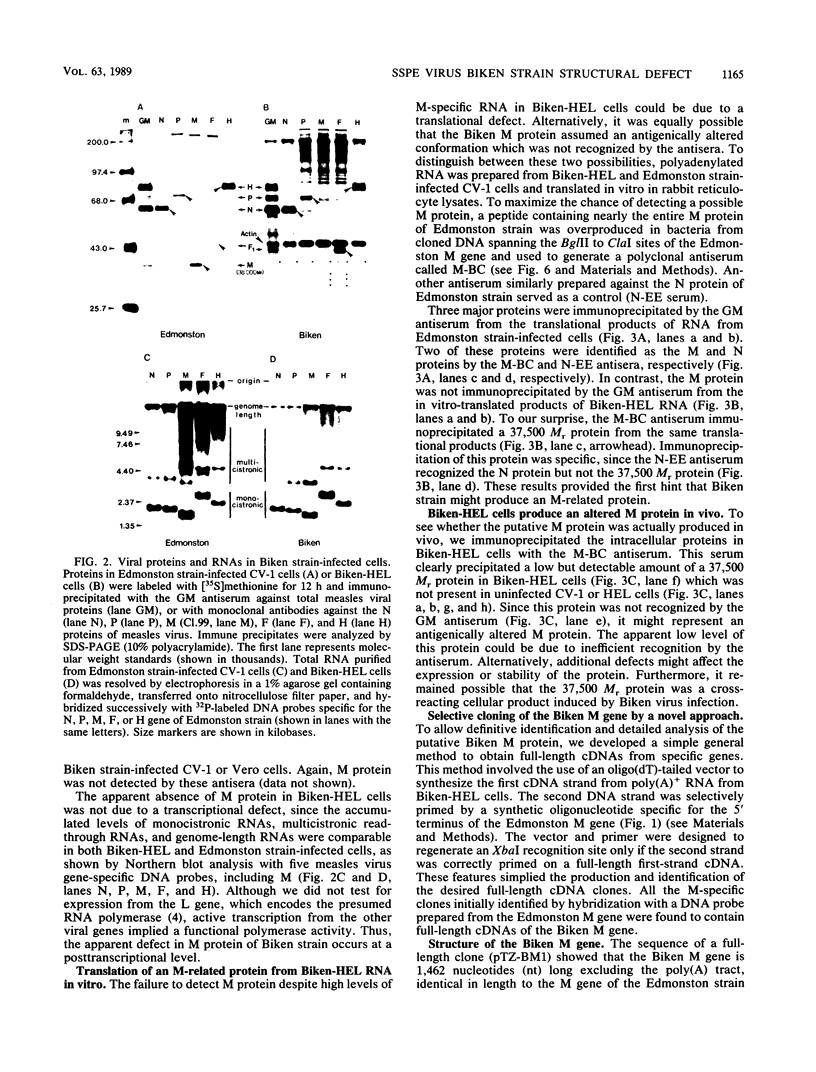
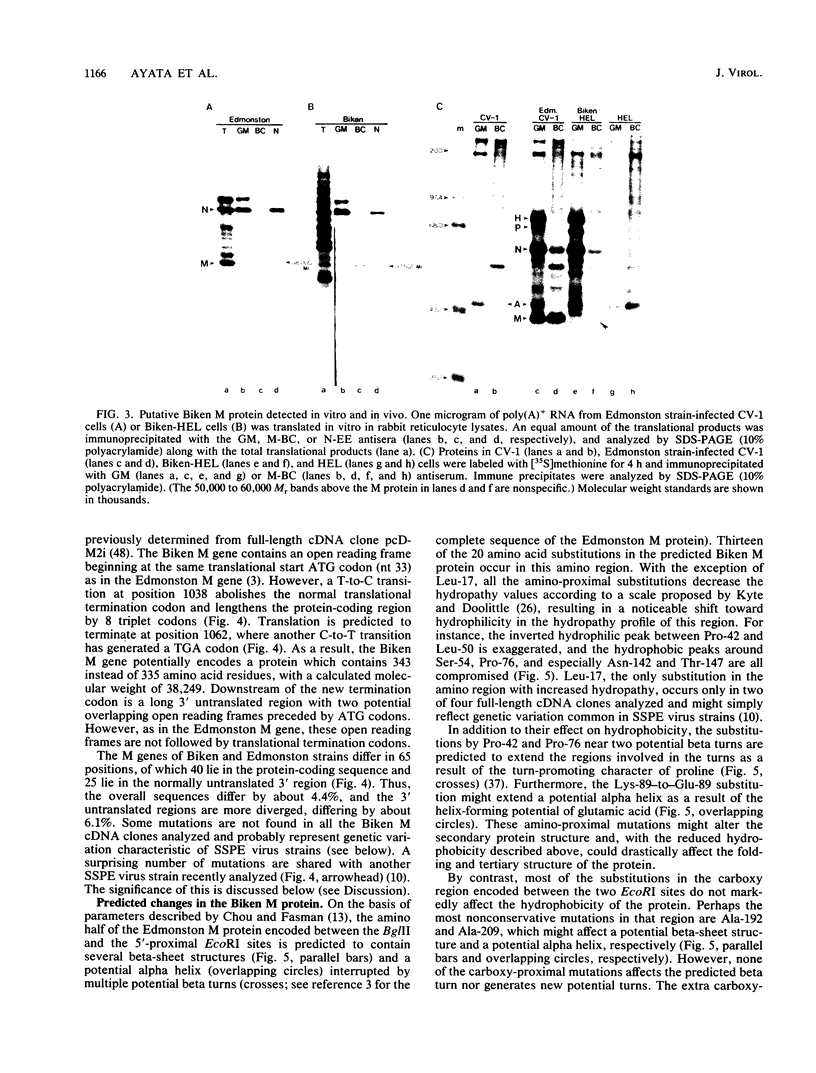
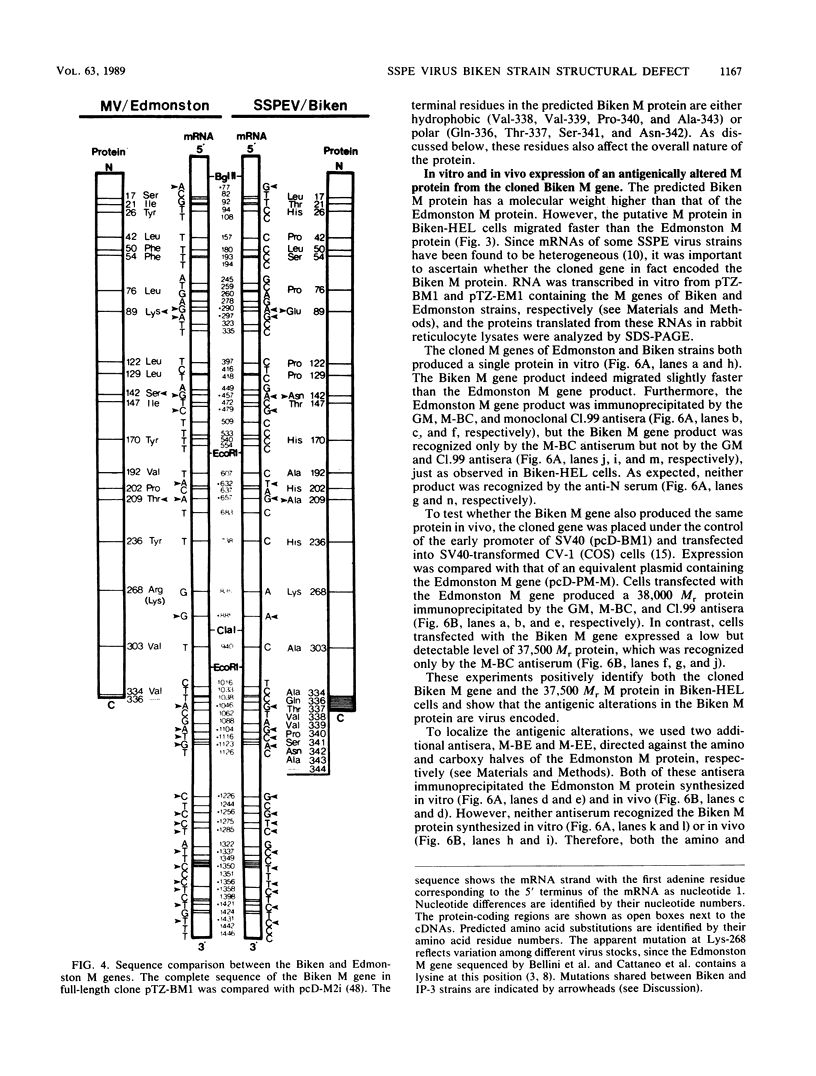
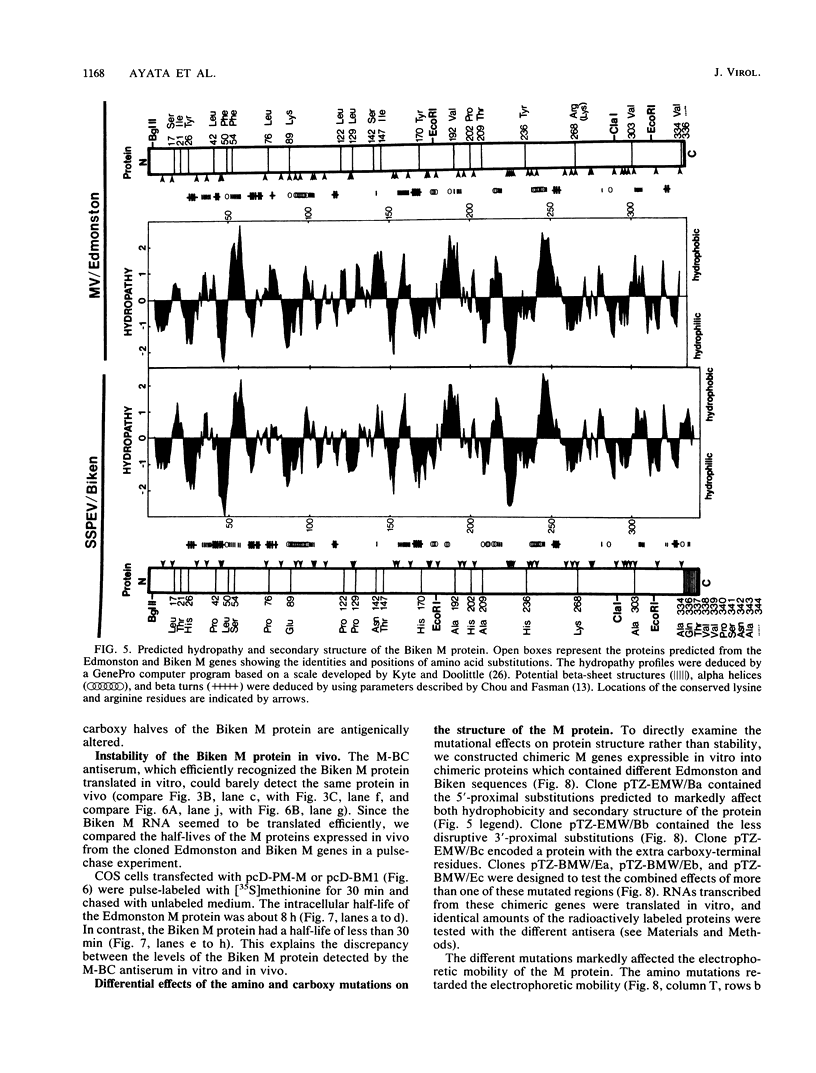
Biken strain, a nonproductive measles viruslike agent isolated from a subacute sclerosing panencephalitis (SSPE) patient, contains a posttranscriptional defect affecting matrix (M) protein. A putative M protein was translated in vitro with RNA from Biken strain-infected cells. A similar protein was detected in vivo by an antiserum against a peptide synthesized from the cloned M gene of Edmonston strain measles virus. By using a novel method, full-length cDNAs of the Biken M gene were selectively cloned. The cloned Biken M gene contained an open reading frame which encoded 8 extra carboxy-terminal amino acid residues and 20 amino acid substitutions predicted to affect both the hydrophobicity and secondary structure of the gene product. The cloned gene was expressed in vitro and in vivo into a 37,500 Mr protein electrophoretically and antigenically distinct from the M protein of Edmonston strain but identical to the M protein in Biken strain-infected cells. Chimeric M proteins synthesized in vitro and in vivo showed that the mutations in the carboxy-proximal region altered the local antigenicity and those in the amino region affected the overall protein conformation. The protein expressed from the Biken M gene was unstable in vivo. Instability was attributed to multiple mutations in both the amino and carboxy regions. A surprising number of mutations in both the coding and noncoding regions of the Biken M gene were identical to those in an independently isolated SSPE virus strain with a similar defect. These results offer insights into the basis of the defect in Biken strain and pose intriguing questions about the evolutionary origins of SSPE viruses in general.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Crowley J. C., Silverman J. I., Menonna J., Cook S. D., Dowling P. C. Measles virus L protein evidences elements of ancestral RNA polymerase. Virology. 1988 Jun;164(2):487–497. doi: 10.1016/0042-6822(88)90563-6. [DOI] [PubMed] [Google Scholar]
- Breschkin A. M., Morgan E. M., McKimm J., Rapp F. SSPE-BIKEN: a naturally arising hemagglutination-defective mutant of measles virus. J Med Virol. 1979;4(1):67–80. doi: 10.1002/jmv.1890040109. [DOI] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Rebmann G., Baczko K., Ter Meulen V., Bellini W. J., Rozenblatt S., Billeter M. A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986 Oct 15;154(1):97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Measles virus and chronic neurological diseases. Ann Neurol. 1981 Jan;9(1):17–20. doi: 10.1002/ana.410090104. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S., McFarland H. F., Mingioli E. S., Sever J. L., McFarlin D. E. Humoral and cellular immune responses to matrix protein of measles virus in subacute sclerosing panencephalitis. J Virol. 1988 Jul;62(7):2483–2489. doi: 10.1128/jvi.62.7.2483-2489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Eble B., Walker D., Stowring L., Ventura P., Blum H., Wietgrefe S., Zupancic M., Tourtellotte W. Natural history of restricted synthesis and expression of measles virus genes in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1985 May;82(9):3020–3024. doi: 10.1073/pnas.82.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Honma H., Maotani K., Ueda S., Kato S., Hirai K. Monoclonal antibodies specific to and cross-reactive with Marek's disease virus and herpesvirus of turkeys. Biken J. 1982 Dec;25(4):171–175. [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Experimental subacute sclerosing panencephalitis: selective disappearance of measles virus matrix protein from the Central nervous system. J Infect Dis. 1981 Aug;144(2):161–169. doi: 10.1093/infdis/144.2.161. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Expression of five viral antigens in cells infected with wild-type and SSPE strains of measles virus: correlation with cytopathic effects and productivity of infections. Arch Virol. 1982;73(3-4):255–262. doi: 10.1007/BF01318079. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature. 1980 Jun 12;285(5765):490–492. doi: 10.1038/285490a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Kristensson K., Brzosko W. J., Kapsenberg J. G. Measles virus matrix protein detected by immune fluorescence with monoclonal antibodies in the brain of patients with subacute sclerosing panencephalitis. J Virol. 1985 Oct;56(1):337–340. doi: 10.1128/jvi.56.1.337-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Tashiro M., Takase S., Homma M. Detection of antibody to M protein of measles virus in patients with subacute sclerosing panencephalitis: a comparative study on immunoprecipitation. Microbiol Immunol. 1985;29(8):709–723. doi: 10.1111/j.1348-0421.1985.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Ohuchi M., Ohuchi R., Homma M. Mode of subacute sclerosing panencephalitis (SSPE) virus infection in tissue culture cells. III. Neurovirulence of cell-free SSPE viruses of Niigata-1, Kitaken-1, and Biken strains. Microbiol Immunol. 1981;25(9):887–893. doi: 10.1111/j.1348-0421.1981.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. K., Koerner T. J., Tzagoloff A. Characterization of a yeast nuclear gene (MST1) coding for the mitochondrial threonyl-tRNA1 synthetase. J Biol Chem. 1985 Dec 5;260(28):15362–15370. [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. A., Fukuda A., Sugiura A. Characterization of major structural proteins of measles virus with monoclonal antibodies. J Gen Virol. 1985 Jul;66(Pt 7):1397–1409. doi: 10.1099/0022-1317-66-7-1397. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Rapid degradation restricts measles virus matrix protein expression in a subacute sclerosing panencephalitis cell line. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7913–7917. doi: 10.1073/pnas.83.20.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormar H., Mehta P. D., Brown H. R. Comparison of wild-type and subacute sclerosing panencephalitis strains of measles virus. Neurovirulence in ferrets and biological properties in cell cultures. J Exp Med. 1978 Sep 1;148(3):674–691. doi: 10.1084/jem.148.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Ueda S., Takahashi M., Kurimura T., Minekawa Y., Suzuki N. Development of extremely attenuated live measles virus vaccine (CAM-EX). Biken J. 1972 Sep;15(3):173–177. [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wechsler S. L., Weiner H. L., Fields B. N. Immune response in subacute sclerosing panencephalitis: reduced antibody response to the matrix protein of measles virus. J Immunol. 1979 Aug;123(2):884–889. [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Functional cDNA library for efficient expression of measles virus-specific gene products in primate cells. J Virol. 1986 Jan;57(1):343–348. doi: 10.1128/jvi.57.1.343-348.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Wipf G., Hirano A. The measles virus matrix gene and gene product defined by in vitro and in vivo expression. Virology. 1987 Apr;157(2):497–508. doi: 10.1016/0042-6822(87)90292-3. [DOI] [PubMed] [Google Scholar]