Abstract
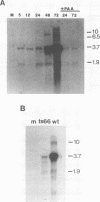
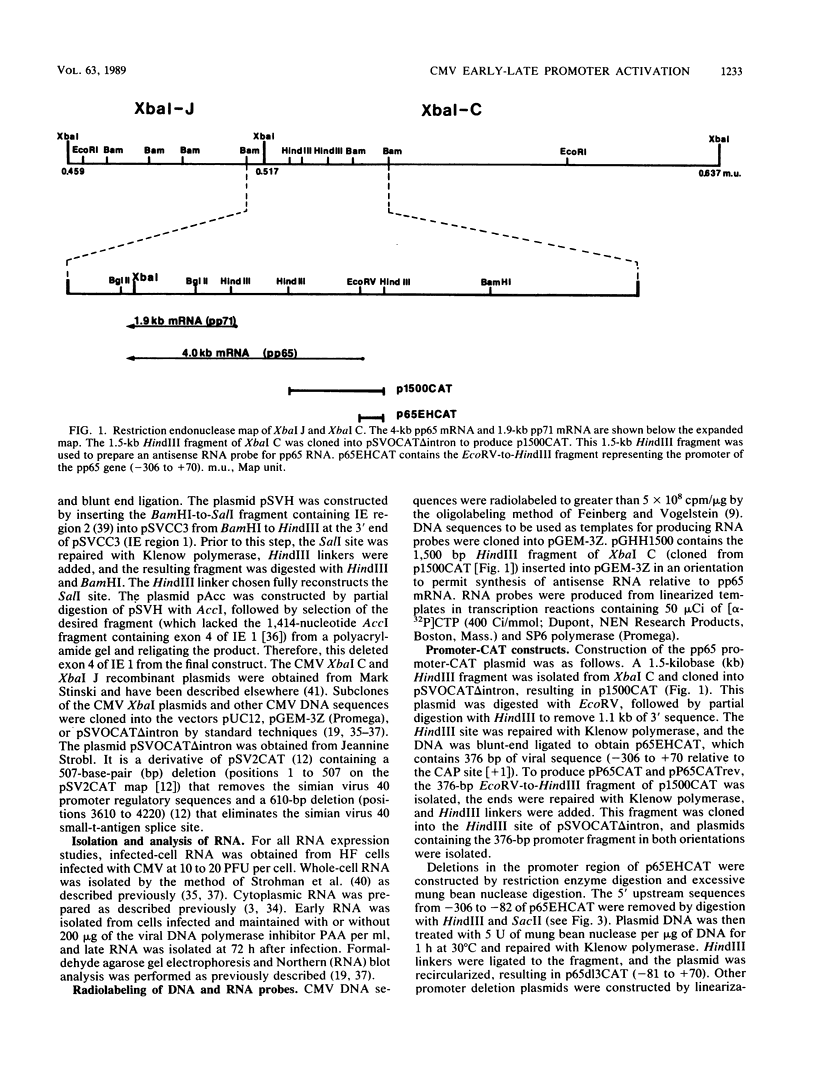
To better understand the regulation of late gene expression in human cytomegalovirus (CMV)-infected cells, we examined expression of the gene that codes for the 65-kilodalton lower-matrix phosphoprotein (pp65). Analysis of RNA isolated at 72 h from cells infected with CMV Towne or ts66, a DNA-negative temperature-sensitive mutant, supported the fact that pp65 is expressed at low levels prior to viral DNA replication but maximally expressed after the initiation of viral DNA replication. To investigate promoter activation in a transient expression assay, the pp65 promoter was cloned into the indicator plasmid containing the gene for chloramphenicol acetyltransferase (CAT). Transfection of the promoter-CAT construct and subsequent superinfection with CMV resulted in activation of the promoter at early times after infection. Cotransfection with plasmids capable of expressing immediate-early (IE) proteins demonstrated that the promoter was activated by IE proteins and that both IE regions 1 and 2 were necessary. Analysis of promoter deletion mutants indicated that the 5' minimal sequence required for activation is -61 from the CAP site (+1) and that an 8-base-pair sequence located at -51 to -58 is necessary for activation of the pp65 promoter. This sequence is repeated once at +93 and is found as an inverted repeat at +67. These studies suggest that interactions between IE proteins and this octamer sequence may be important for the regulation and expression of this CMV gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Keller W., Dale T., Schöler H. R., Tebb G., Mattaj I. W. A transcription factor which binds to the enhancers of SV40, immunoglobulin heavy chain and U2 snRNA genes. Nature. 1987 Jan 15;325(6101):268–272. doi: 10.1038/325268a0. [DOI] [PubMed] [Google Scholar]
- Campos R., Villarreal L. P. An SV40 deletion mutant accumulates late transcripts in a paranuclear extract. Virology. 1982 May;119(1):1–11. doi: 10.1016/0042-6822(82)90059-9. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Huang E. S. Nucleotide sequence of a human cytomegalovirus DNA fragment encoding a 67-kilodalton phosphorylated viral protein. J Virol. 1985 Oct;56(1):7–11. doi: 10.1128/jvi.56.1.7-11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Schmidt C. A., Kaplan A. S. Patterns of transcription of human cytomegalovirus in permissively infected cells. J Virol. 1980 Aug;35(2):277–286. doi: 10.1128/jvi.35.2.277-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Mocikat R., Zachau H. G. Sequences closely related to an immunoglobulin gene promoter/enhancer element occur also upstream of other eukaryotic and of prokaryotic genes. Nucleic Acids Res. 1986 Nov 25;14(22):8819–8827. doi: 10.1093/nar/14.22.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins W. F., Stinski M. F. Expression of a human cytomegalovirus late gene is posttranscriptionally regulated by a 3'-end-processing event occurring exclusively late after infection. Mol Cell Biol. 1986 Dec;6(12):4202–4213. doi: 10.1128/mcb.6.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Wilkinson G. W. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987 Feb;7(1):17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Jahn G., Bürkle A., Freese U. K., Fleckenstein B., zur Hausen H. Genomic localization, sequence analysis, and transcription of the putative human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):119–124. doi: 10.1128/jvi.61.1.119-124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston T. W., Malone C. L., Witte P. R., Stinski M. F. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J Virol. 1987 Oct;61(10):3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Large-scale rearrangement of homologous regions in the genomes of HCMV and EBV. Virology. 1987 Apr;157(2):397–413. doi: 10.1016/0042-6822(87)90282-0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Staprans S. I., Spector D. H. Analysis of the major transcripts encoded by the long repeat of human cytomegalovirus strain AD169. J Virol. 1985 Mar;53(3):711–718. doi: 10.1128/jvi.53.3.711-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B., Gmeiner A., Sarnow P., Levine A. J., Fleckenstein B. Physical mapping of human cytomegalovirus genes: identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide. Virology. 1984 Apr 15;134(1):91–102. doi: 10.1016/0042-6822(84)90275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande H., Baak S. W., Riggs A. D., Clark B. R., Shively J. E., Zaia J. A. Cloning and physical mapping of a gene fragment coding for a 64-kilodalton major late antigen of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4965–4969. doi: 10.1073/pnas.81.15.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mutul J., Macchi M., Wasylyk B. Mutational analysis of the contribution of sequence motifs within the IgH enhancer to tissue specific transcriptional activation. Nucleic Acids Res. 1988 Jul 11;16(13):6085–6096. doi: 10.1093/nar/16.13.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri P., Rooney R., Nevins J. R. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987 Dec 20;6(13):4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Thimmappaya B. Two promoter-specific host factors interact with adjacent sequences in an EIA-inducible adenovirus promoter. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6112–6116. doi: 10.1073/pnas.84.17.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Stinski M. F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985 Dec;56(3):676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]